Abstract
Background
Sarcopenia indicates poor prognosis in various malignancies. We evaluated the association of sarcopenia with overall (OS) and progression-free survival (PFS) in metastatic esophageal cancer (MEC) patients, a population often presenting with poor nutritional status.
Methods
In newly diagnosed MEC patients managed at the Princess Margaret (PM) Cancer Centre (diagnosed 2006–2015), total muscle area, visceral adiposity (VA), and subcutaneous adiposity (SA) were quantified on abdominal computed tomography at L3. Sarcopenia was determined using published cutoffs, based on sex and height.
Results
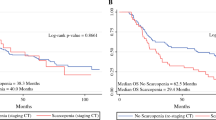
Of 202 MEC patients, most were male (166/82%), < 65 years (116/57%), and had adenocarcinoma histology (141/70%); 110/54% had recurrent MEC after initial curative-intent treatment; 92/46% presented with de novo MEC. At stage IV diagnosis, 20/10% were underweight, 97/48% were normal-weight and 84/42% were overweight/obese; 103/51% were sarcopenic. Sarcopenia was associated with worse median OS (4.6 vs. 7.9 months; log-rank p = 0.03) and 1-year survival, even after adjusting for other body composition variables (e.g., BMI, VA, and SA): adjusted-HR 1.51 [95% CI 1.1–2.2, p = 0.02]. In post hoc analysis, sarcopenia was highly prognostic in adenocarcinomas (p = 0.003), but not squamous cell carcinomas (SCC). In patients receiving palliative systemic treatment (104/51%), sarcopenia was associated with shorter PFS (p = 0.004) in adenocarcinoma patients (75/72%).
Conclusions
In metastatic esophageal adenocarcinomas, sarcopenia is associated with worse PFS and OS. In metastatic esophageal SCC, there was a non-significant trend for worse PFS but no association with OS. In order to offset the poor prognosis associated with sarcopenia particularly in metastatic esophageal adenocarcinoma patients, future research should focus on possible countermeasures.


Similar content being viewed by others
Data Availability
The raw data used in this study may be requested from the authors.
References
Haj Mohammad N, Bernards N, van Putten M, Lemmens V, van Oijen MGH, van Laarhoven HWM. Volume-outcome relation in palliative systemic treatment of metastatic oesophagogastric cancer. Eur J Cancer. 2017;78:28–36.
Gangadharan A, Choi SE, Hassan A, Ayoub NM, Durante G, Balwani S, et al. Protein calorie malnutrition, nutritional intervention and personalized cancer care. Oncotarget. 2017;8(14):24009–30.
Baracos VE. Cancer-associated malnutrition. Eur J Clin Nutr. 2018;72(9):1255–9.
Blum D, Omlin A, Baracos VE, Solheim TS, Tan BH, Stone P, et al. Cancer cachexia: a systematic literature review of items and domains associated with involuntary weight loss in cancer. Crit Rev Oncol Hematol. 2011;80(1):114–44.
Duan XF, Tang P, Shang XB, Jiang HJ, Zhao Q, Yu ZT. High body mass index worsens survival in patients with esophageal squamous cell carcinoma after esophagectomy. Dig Surg. 2017;34(4):319–27.
Mengardo V, Pucetti F, Mc Cormack O, Chaudry A, Allum WH. The impact of obesity on esophagectomy: a meta-analysis. Dis Esophagus. 2018;31(6):dox149.
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–95.
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31.
Bozzetti F. Forcing the vicious circle: sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann Oncol. 2017;28(9):2107–18.
Cespedes Feliciano EM, Popuri K, Cobzas D, Baracos VE, Beg MF, Khan AD, et al. Evaluation of automated computed tomography segmentation to assess body composition and mortality associations in cancer patients. J Cachexia Sarcopenia Muscle. 2020;11(5):1258–69.
Kudou K, Saeki H, Nakashima Y, Edahiro K, Korehisa S, Taniguchi D, et al. Prognostic significance of sarcopenia in patients with esophagogastric junction cancer or upper gastric cancer. Ann Surg Oncol. 2017;24(7):1804–10.
Paireder M, Asari R, Kristo I, Rieder E, Tamandl D, Ba-Ssalamah A, et al. Impact of sarcopenia on outcome in patients with esophageal resection following neoadjuvant chemotherapy for esophageal cancer. Eur J Surg Oncol. 2017;43(2):478–84.
Sato S, Kunisaki C, Suematsu H, Tanaka Y, Miyamoto H, Kosaka T, et al. Impact of sarcopenia in patients with unresectable locally advanced esophageal cancer receiving chemoradiotherapy. In Vivo. 2018;32(3):603–10.
Matsunaga T, Miyata H, Sugimura K, Motoori M, Asukai K, Yanagimoto Y, et al. Prognostic significance of sarcopenia and systemic inflammatory response in patients with esophageal cancer. Anticancer Res. 2019;39(1):449–58.
Tan BH, Brammer K, Randhawa N, Welch NT, Parsons SL, James EJ, et al. Sarcopenia is associated with toxicity in patients undergoing neo-adjuvant chemotherapy for oesophago-gastric cancer. Eur J Surg Oncol. 2015;41(3):333–8.
Kurk SA, Peeters PHM, Dorresteijn B, de Jong PA, Jourdan M, Kuijf HJ, et al. Impact of different palliative systemic treatments on skeletal muscle mass in metastatic colorectal cancer patients. J Cachexia Sarcopenia Muscle. 2018;9(5):909–19.
https://tomovision.com/Sarcopenia_Help/index.htm. Alberta Protocol Link. Accessed 12 Mar 2022.
Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31(12):1539–47.
Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629–35.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–26.
Dijksterhuis WPM, Pruijt MJ, van der Woude SO, Klaassen R, Kurk SA, van Oijen MGH, et al. Association between body composition, survival, and toxicity in advanced esophagogastric cancer patients receiving palliative chemotherapy. J Cachexia Sarcopenia Muscle. 2019;10(1):199–206.
Ma LX, Taylor K, Espin-Garcia O, Anconina R, Suzuki C, Allen MJ, et al. Prognostic significance of nutritional markers in metastatic gastric and esophageal adenocarcinoma. Cancer Med. 2021;10(1):199–207.
Hayashi N, Ando Y, Gyawali B, Shimokata T, Maeda O, Fukaya M, et al. Low skeletal muscle density is associated with poor survival in patients who receive chemotherapy for metastatic gastric cancer. Oncol Rep. 2016;35(3):1727–31.
Blauwhoff-Buskermolen S, Versteeg KS, de van der Schueren MA, den Braver NR, Berkhof J, Langius JA, et al. Loss of muscle mass during chemotherapy is predictive for poor survival of patients with metastatic colorectal cancer. J Clin Oncol. 2016;34(12):1339–44.
Jarvinen T, Ilonen I, Kauppi J, Salo J, Rasanen J. Loss of skeletal muscle mass during neoadjuvant treatments correlates with worse prognosis in esophageal cancer: a retrospective cohort study. World J Surg Oncol. 2018;16(1):27.
Anconina R, Ortega C, Metser U, Liu ZA, Suzuki C, McInnis M, et al. Influence of sarcopenia, clinical data, and 2-[(18)F] FDG PET/CT in outcome prediction of patients with early-stage adenocarcinoma esophageal cancer. Eur J Nucl Med Mol Imaging. 2021;49:1012–20.
Harada K, Ida S, Baba Y, Ishimoto T, Kosumi K, Tokunaga R, et al. Prognostic and clinical impact of sarcopenia in esophageal squamous cell carcinoma. Dis Esophagus. 2016;29(6):627–33.
Cancer Genome Atlas Research N, Analysis Working Group, Asan U, Agency BCC, Brigham WH, Broad I, et al. Integrated genomic characterization of oesophageal carcinoma. Nature. 2017;541(7636):169–75.
Veugelers PJ, Porter GA, Guernsey DL, Casson AG. Obesity and lifestyle risk factors for gastroesophageal reflux disease, Barrett esophagus and esophageal adenocarcinoma. Dis Esophagus. 2006;19(5):321–8.
Xishan Z, Ye Z, Feiyan M, Liang X, Shikai W. The role of prognostic nutritional index for clinical outcomes of gastric cancer after total gastrectomy. Sci Rep. 2020;10(1):17373.
Zheng Z, Zshu H, Cai H. Preoperative prognostic nutritional index predict survival in patients with resectable esophageal squamous cell carcinoma. Front Nutr. 2022;13(9): 824839.
Acknowledgements
Grant Support: Funding for this study was provided by the Lusi Wong Family Fund and the Alan Brown Chair.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or substitute for it was obtained from all patients for being included in the study.
Conflict of interest
On behalf of the co-authors of this manuscript, we declare the following conflicts of interests: Sabine Schmid: Grants or contracts from any entity (all institutional): Von Tobel Stiftung, Janssen, AstraZeneca, BMS; Fill the Gap (University of Zürich); Swiss Cancer League foundation. Advisory boards (all institutional): AstraZeneca, BMS, MSD. Dmitry Rozenberg: all support for the present manuscript: salary support for Sandra Faire and Ivan Fecan Professorship. The following authors declare no conflicts of interest: Ulf Zeuge, Aline F. Fares, Joelle Soriano, Katrina Hueniken, Jaspreet Bajwa, Wanning Wang, Sarah Rudolph-Naiberg, M. Catherine Brown, Jonathan Yeung, M. Catherine Brown, Eric X. Chen, Raymond W. Jang, Wei Xu, Elena Elimova, Geoffrey Liu, and Micheal C. McInnis.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10388_2022_981_MOESM1_ESM.docx
Supplementary file1 Supplementary Tab.1 Palliative treatment details of full cohort (left) and by sarcopenia status (right). Palliative treatments at UHN or affiliated hospitals. PNI, prognostic nutritional index (around time of baseline CT); SD, standard deviation (DOCX 30 KB)
10388_2022_981_MOESM2_ESM.docx
Supplementary file2 Supplementary Tab.2 Overall survival in adenocarcinoma patients: Univariable and multivariable Cox proportional hazards regression, associations between sarcopenia and overall survival. Abbreviations: BMI, Body mass index; ECOG, Eastern Cooperative Oncology Group; VA, visceral adipose tissue; SA, subcutaneous adipose tissue. †P-values computed from Wald test for global statistical significance of the indicated variable (DOCX 30 KB)
10388_2022_981_MOESM3_ESM.docx
Supplementary file3 Supplementary Tab.3 Overall survival in SCC patients: Univariable and multivariable Cox proportional hazards regression, associations between sarcopenia and overall survival. Abbreviations: BMI, Body mass index; ECOG, Eastern Cooperative Oncology Group; VA, visceral adipose tissue; SA, subcutaneous adipose tissue. †P-values computed from Wald test for global statistical significance of the indicated variable (DOCX 29 KB)
10388_2022_981_MOESM4_ESM.docx
Supplementary file4 Supplementary Fig.1 Body compositions at the level of L3 in three exemplary patients. Skeletal muscle is outlined (red) and psoas muscle (blue). Subcutaneous adipose tissue (cyan) and visceral adipose tissue (yellow)(a): SMI=39.8cm2/m2, VA=15.2cm2 SA=149cm2, BMI=31; (b): SMI=34.2cm2/m2, VA=28cm2 SA=28cm2, BMI=16.5; (c): SMI=72cm2/m2, VA=491cm2, SA 196cm2, BMI=36 (DOCX 487 KB)
10388_2022_981_MOESM6_ESM.docx
Supplementary file6 Supplementary Fig3 Relationship between the sarcopenia status and different body composition metrics. These metrics are all at the third lumbar vertebra. Top: (total) muscle area (MA; Panel a), subcutaneous adipose tissue (SA; Panel b), Body Mass Index (BMI; Panel c), and visceral adipose tissue (VA; Panel d). Bottom: Pairwise Pearson correlations of the four body composition metrics. Abbreviations: rho = Spearman’s rho correlation coefficient for the correlation between each X and Y variable (DOCX 391 KB)
10388_2022_981_MOESM7_ESM.docx
Supplementary file7 Supplementary Fig4 Overall survival (left) and progression free survival (right) by sarcopenia status and histology. Adenocarcinoma (a and b) and squamous cell carcinoma (c and d). Overall survival in SCC adds up to 52 patients, because one sarcopenic patient was missing BMI for calculations. Abbreviations: SCC, Squamous cell carcinoma. Note that in this analysis, sarcopenic non-overweight/obese refers to individuals who are sarcopenic but with BMI < 25 mg/m2; sarcopenic overweight/obese is defined as sarcopenia in individuals who have BMI > 25mg/m2; non-sarcopenic refers to all patients who do not meet the definition of sarcopenia used throughout this study (DOCX 279 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zeuge, U., Fares, A.F., Soriano, J. et al. Differential prognostic significance of sarcopenia in metastatic esophageal squamous and adenocarcinoma. Esophagus 20, 557–566 (2023). https://doi.org/10.1007/s10388-022-00981-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10388-022-00981-y