Abstract
Background
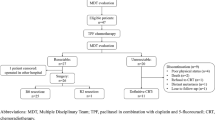
Definitive chemoradiotherapy is the standard of care for T4 and/or M1 lymph node cancers. Although the treatment strategy would depend mainly on the diagnosis of T4 disease, the diagnosis of T disease is not standardized and cases of borderline resectable T4 disease are often encountered. We have administered the triplet regimen as intensive chemotherapy for cases of borderline resectable T4 disease.
Methods
We reviewed the records of patients with esophageal cancer who were treated between August 2009 and August 2010 at the Tochigi Cancer Center. Our treatment strategy for clinical stage II/III disease with unequivocal T4 disease in an adjacent organ was definitive chemoradiotherapy. Cases of clinical stage II/III disease with borderline resectable T4 disease were treated with preoperative chemotherapy via the triplet regimen of docetaxel and cisplatin plus 5-fluorouracil (DCF) followed by esophagectomy.
Results
Nine patients were treated with preoperative chemotherapy. Six patients with defined borderline T4 disease were treated with DCF. DCF toxicities were tolerable and all patients underwent subsequent surgery. The R0 resection rate was 66 % (4/6), although pathological T4 disease was found in 50 % of patients (3/6). There were no major complications or mortality, although the median blood loss and operation time were relatively higher with this regimen. Although 5 patients died of recurrence or coexisting disease, oral intake was maintained at the terminal stage.
Conclusion
Preoperative DCF followed by surgery seems to be a good option for select patients with T4 disease. Further investigations are warranted and a well-designed prospective trial is needed to draw a conclusion.

Similar content being viewed by others
References
Herskovic A, Martz K, Al-Sarraf M, Leichman L, Brindle J, Vaitkevicius V, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326(24):1593–8.
Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA, Al-Sarraf M, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). JAMA. 1999;281(17):1623–7.
Al-Sarraf M, Martz K, Herskovic A, Leichman L, Brindle J, Vaitkevicius V, et al. Progress report of combined chemoradiotherapy versus radiotherapy alone in patients with esophageal cancer: an intergroup study. J Clin Oncol. 1997;15(1):277–84.
Ohtsu A, Boku N, Muro K, Chin K, Muto M, Yoshida S, et al. Definitive chemoradiotherapy for T4 and/or M1 lymph node squamous cell carcinoma of the esophagus. J Clin Oncol. 1999;17:2915–21.
Shinoda M, Ando N, Kato H. A multicenter randomized phase II/III study comparing concurrent chemoradiotherapy (CRT) with low-dose cisplatin plus continuous infusion of 5-fluorouracil (LDPF) and standard-dose PF (SDPF) for locally advanced unresectable squamous cell carcinoma of the thoracic esophagus (JCOG 0303). ASCO Annual Meeting Proceeding 2010; abstract 4053.
Nakamura T, Ota M, Ohki T, Sato T, Shirai Y, Yamamoto M, et al. Induction chemoradiotherapy followed by esophagectomy for advanced squamous cell carcinoma of the esophagus. Esophagus. 2011;8(2):89–95.
Yano M, Tsujinaka T, Shiozaki H, Inoue M, Doki Y, Yamamoto M, et al. Concurrent chemotherapy (5-fluorouracil and cisplatin) and radiation therapy followed by surgery for T4 squamous cell carcinoma of the esophagus. J Surg Oncol. 1999;70(1):25–32.
Akutsu Y, Matsubara H, Shuto K, Uesato M, Mori M, Hoshino I, et al. Clinical and pathologic evaluation of the effectiveness of neoadjuvant chemoradiation therapy in advanced esophageal cancer patients. World J Surg. 2009;33(5):1002–9.
Bedenne L, Michel P, Bouché O, Milan C, Mariette C, Conroy T, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25(10):1160–8.
Stahl M, Stuschke M, Lehmann N, Meyer HJ, Walz MK, Seeber S, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23(10):2310–7.
Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–15.
Vermorken JB, Remenar E, van Herpen C, Gorlia T, Sc M, Mesia R, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695–704.
Ferri LE, Ades S, Alcindor T, Chasen M, Marcus V, Hickeson M, et al. Perioperative docetaxel, cisplatin, and 5-fluorouracil (DCF) for locally advanced esophageal and gastric adenocarcinoma: a multicenter phase II trial. Ann Oncol. 2011;23(6):1512–7.
Bayraktar UD, Bayraktar S, Hosein P, Chen E, Koniaris LG, Max C, et al. Preoperative docetaxel/cisplatin/5-fluorouracil chemotherapy in patients with locally advanced gastro-esophageal adenocarcinoma. Med Oncol. 2011;29(3):1707–10.
Hara H, Daiko H, Kato K, Igaki H, Kadowaki S, Tanaka Y, et al. Final results of a feasibility study of neoadjuvant chemotherapy with docetaxel, cisplatin, and fluorouracil (DCF) for clinical stage II/III esophageal squamous cell carcinoma. Proceeding ASCO 2011; abstract 4060.
Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–7.
Sobin LH, Wittedkind CH. UICC TNM classification of malignant tumors. 6th ed. New York: Wiley-Liss, Inc.; 2002. p. 60–4.
Japanese Society for Esophageal Diseases: guidelines for clinical and pathologic studies on carcinoma of the esophagus, ninth edition: Preface, general principles, part II. Esophagus 2004;1:107–125.
Urba S, Wolf G, Eisbruch A, Worden F, Lee J, Bradford C, et al. Single-cycle induction chemotherapy selects patients with advanced laryngeal cancer for combined chemoradiation: a new treatment paradigm. J Clin Oncol. 2006;24:593–8.
Ethical statement
This article does not contain any studies with human or animal subjects performed by any author(s).
Conflict of interest
All authors have declared no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamamoto, Y., Onodera, K., Saito, M. et al. Case series of preoperative triplet-regimen (docetaxel, CDDP and 5-fluorouracil) for borderline resectable T4 thoracic esophageal carcinoma. Esophagus 10, 205–210 (2013). https://doi.org/10.1007/s10388-013-0384-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10388-013-0384-6