Abstract
Purpose
To assess the long-term safety and efficacy of omidenepag isopropyl (OMDI) 0.002% (a first-in-class, selective, non-prostaglandin, prostanoid EP2 receptor agonist), alone or administered concomitantly with timolol 0.5%, in patients with open-angle glaucoma (OAG, including normal-tension and exfoliation glaucoma) or ocular hypertension (OHT).
Study design
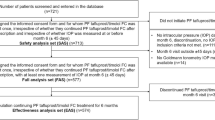
Open-label, multicenter, Phase 3 study (NCT02822729).
Methods
Patients aged ≥ 20 years, with OAG or OHT, and a baseline diurnal intraocular pressure (IOP) ≥ 16– < 22 mmHg (Group 1) or ≥ 22– ≤ 34 mmHg (Groups 2 and 3) were enrolled. All patients (N = 125) received OMDI 0.002% once daily. Group 3 also received timolol 0.5% twice daily. IOP was measured at baseline and at Weeks 2, 4, 8, 12, 26, 40, and 52.
Results
Significant reductions in mean diurnal IOP from baseline occurred at every visit (P < 0.0001). Mean ± SE diurnal IOP reduction at Week 52 was −3.7 ± 0.3 mmHg (Group 1), −5.6 ± 0.5 mmHg (Group 2), and −8.4 ± 0.6 mmHg (Group 3). Most adverse events (AEs) were mild, and no serious treatment-related AEs were reported. Conjunctival hyperemia (incidence: monotherapy [Groups 1 and 2], 18.8%; concomitant [Group 3], 45.0%) and macular edema (ME)/cystoid macular edema (CME) (incidence: monotherapy, 11.8%; concomitant, 15.0%) occurred most frequently. All treatment-related ME/CME cases occurred in pseudophakic eyes and responded to standard-of-care treatment and study drug discontinuation.
Conclusions
In this study, OMDI 0.002%, alone or administered concomitantly with timolol 0.5%, resulted in sustained IOP reduction over 52 weeks in patients with OAG or OHT. Concomitant treatment resulted in increased efficacy and increased incidence of conjunctival hyperemia.



Similar content being viewed by others
References
European Glaucoma Society. European Glaucoma Society terminology and guidelines for glaucoma (4th Edn)—Chapter 2: Classification and terminology. Br J Ophthalmol. 2017;101:73–127.
Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7.
Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–90.
Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998;126:487–97.
Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–79.
Garway-Heath DF, Crabb DP, Bunce C, Lascaratos G, Amalfitano F, Anand N, et al. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet. 2015;385:1295–304.
European Glaucoma Society. European Glaucoma Society terminology and guidelines for glaucoma (4th Edn)—Chapter 3: Treatment principles and options. Br J Ophthalmol. 2017;101:130–95.
Honrubia F, García-Sánchez J, Polo V, de la Casa JM, Soto J. Conjunctival hyperaemia with the use of latanoprost versus other prostaglandin analogues in patients with ocular hypertension or glaucoma: a meta-analysis of randomised clinical trials. Br J Ophthalmol. 2009;93:316–21.
Inoue K, Shiokawa M, Higa R, Sugahara M, Soga T, Wakakura M, et al. Adverse periocular reactions to five types of prostaglandin analogs. Eye (Lond). 2012;26:1465–72.
Inoue K, Setogawa A, Tomita G. Nonresponders to prostaglandin analogs among normal-tension glaucoma patients. J Ocul Pharmacol Ther. 2016;32:90–6.
Brennan N, Dehabadi MH, Nair S, Quartilho A, Bunce C, Reekie I, et al. Efficacy and safety of bimatoprost in glaucoma and ocular hypertension in non-responder patients. Int J Ophthalmol. 2017;10:1251–4.
Schmier JK, Lau EC, Covert DW. Two-year treatment patterns and costs in glaucoma patients initiating treatment with prostaglandin analogs. Clin Ophthalmol. 2010;4:1137–43.
Fuwa M, Toris CB, Fan S, Taniguchi T, Ichikawa M, Odani-Kawabata N, et al. Effects of a novel selective EP2 receptor agonist, omidenepag isopropyl, on aqueous humor dynamics in laser-induced ocular hypertensive monkeys. J Ocul Pharmacol Ther. 2018;34:531–7.
Kirihara T, Taniguchi T, Yamamura K, Iwamura R, Yoneda K, Odani-Kawabata N, et al. Pharmacologic characterization of omidenepag isopropyl, a novel selective EP2 receptor agonist, as an ocular hypotensive agent. Invest Ophthalmol Vis Sci. 2018;59:145–53.
Santen Pharmaceutical Co., Ltd. EYBELIS® ophthalmic solution 0.002% package insert [Internet]. 2018 [cited 2020 Sep 4]. https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/300237_1319764Q1027_1_05 (in Japanese)
Santen Pharmaceutical Co., Ltd. Eybelis ophthalmic solution 0.002% package insert [Internet]. [cited 2021 Jan 26]. https://nedrug.mfds.go.kr/pbp/CCBBB01/getItemDetail?itemSeq=201908209 (in Korean)
Santen Pharmaceutical Co., Ltd. Eybelis Ophthalmic Solution 0.002% package insert [Taiwan] [Internet]. [cited 2021 Jan 26]. https://info.fda.gov.tw/MLMS/H0001D3.aspx?LicId=52027906 (in Chinese)
Santen Pharmaceutical Co., Ltd. Eybelis ophthalmic solution 0.002% package insert [Thailand] [Internet]. [cited 2021 Jan 26]. https://porta.fda.moph.go.th/FDA_SEARCH_ALL/MAIN/SEARCH_CENTER_MAIN (in Thai)
Crawford K, Kaufman PL. Pilocarpine antagonizes prostaglandin F2α-induced ocular hypotension in monkeys: evidence for enhancement of uveoscleral outflow by prostaglandin F2α. Arch Ophthalmol. 1987;105:1112–6.
Cheema A, Chang RT, Shrivastava A, Singh K. Update on the medical treatment of primary open-angle glaucoma. Asia Pac J Ophthalmol (Phila). 2016;5:51–8.
Aihara M, Lu F, Kawata H, Iwata A, Odani-Kawabata N, Shams NK. Omidenepag isopropyl versus latanoprost in primary open-angle glaucoma and ocular hypertension: the phase 3 AYAME study. Am J Ophthalmol. 2020;220:53–63.
Aihara M, Ropo A, Lu F, Kawata H, Iwata A, Odani-Kawabata N, et al. Intraocular pressure-lowering effect of omidenepag isopropyl in latanoprost non-/low-responder patients with primary open-angle glaucoma or ocular hypertension: the FUJI study. Jpn J Ophthalmol. 2020;64:398–406.
Aihara M, Lu F, Kawata H, Tanaka Y, Yamamura K, Odani-Kawabata N, et al. Pharmacokinetics, safety, and intraocular pressure-lowering profile of omidenepag isopropyl, a selective, nonprostaglandin, prostanoid EP2 receptor agonist, in healthy Japanese and Caucasian volunteers (Phase I study). J Ocul Pharmacol Ther. 2019;35:542–50.
Koseki N, Araie M, Shirato S, Yamamoto S. Effect of trabeculectomy on visual field performance in central 30 degrees field in progressive normal-tension glaucoma. Ophthalmology. 1997;104:197–201.
Kim M, Kim DM, Park KH, Kim T-W, Jeoung JW, Kim SH. Intraocular pressure reduction with topical medications and progression of normal-tension glaucoma: a 12-year mean follow-up study. Acta Ophthalmol. 2013;91:e270–5.
DuBiner H, Cooke D, Dirks M, Stewart WC, VanDenburgh AM, Felix C. Efficacy and safety of bimatoprost in patients with elevated intraocular pressure: a 30-day comparison with latanoprost. Semin Ophthalmol. 2001;45(Suppl. 4):S353–60.
Cantor LB, Hoop J, Morgan L, Wudunn D, Catoria Y, Bimatoprost-Travoprost Study Group. Intraocular pressure-lowering efficacy of bimatoprost 0.03% and travoprost 0.004% in patients with glaucoma or ocular hypertension. Br J Ophthalmol. 2006;90:1370–3.
Mishra D, Sinha BP, Kumar MS. Comparing the efficacy of latanoprost (0.005%), bimatoprost (0.03%), travoprost (0.004%), and timolol (0.5%) in the treatment of primary open angle glaucoma. Korean J Ophthalmol. 2014;28:399–407.
Chen R, Yang K, Zheng Z, Ong ML, Wang NL, Zhan SY. Meta-analysis of the efficacy and safety of latanoprost monotherapy in patients with angle-closure glaucoma. J Glaucoma. 2016;25:e134–44.
Takagi Y, Santo K, Hashimoto M, Fukuchi T. Ocular hypotensive effects of prostaglandin analogs in Japanese patients with normal-tension glaucoma: a literature review. Clin Ophthalmol. 2018;12:1837–44.
Watanabe K, Chiou GC. Action mechanism of timolol to lower the intraocular pressure in rabbits. Ophthalmic Res. 1983;15:160–7.
Alm A, Grierson I, Shields MB. Side effects associated with prostaglandin analog therapy. Surv Ophthalmol. 2008;53(Suppl. 1):S93-105.
Terao E, Nakakura S, Fujisawa Y, Nagata Y, Ueda K, Kobayashi Y, et al. Time course of conjunctival hyperemia induced by omidenepag isopropyl ophthalmic solution 0.002%: a pilot, comparative study versus ripasudil 0.4. BMJ Open Ophthalmol. 2020;5: e000538.
Liu AW, Gan LY, Yao X, Zhou J. Long-term assessment of prostaglandin analogs and timolol fixed combinations vs prostaglandin analogs monotherapy. Int J Ophthalmol. 2016;9:750–6.
Quaranta L, Biagioli E, Riva I, Rulli E, Poli D, Katsanos A, et al. Prostaglandin analogs and timolol-fixed versus unfixed combinations or monotherapy for open-angle glaucoma: a systematic review and meta-analysis. J Ocul Pharmacol Ther. 2013;29:382–9.
Rotsos TG, Moschos MM. Cystoid macular edema. Clin Ophthalmol. 2008;2:919–30.
Henderson BA, Kim JY, Ament CS, Ferrufino-Ponce ZK, Grabowska A, Cremers SL. Clinical pseudophakic cystoid macular edema. Risk factors for development and duration after treatment. J Cataract Refract Surg. 2007;33:1550–8.
Wendel C, Zakrzewski H, Carleton B, Etminan M, Mikelberg FS. Association of postoperative topical prostaglandin analog or beta-blocker use and incidence of pseudophakic cystoid macular edema. J Glaucoma. 2018;27:402–6.
Holló G, Aung T, Cantor LB, Aihara M. Cystoid macular edema related to cataract surgery and topical prostaglandin analogs: mechanism, diagnosis, and management. Surv Ophthalmol. 2020;65:496–512.
Furuichi M, Chiba T, Abe K, Kogure S, Iijima H, Tsukahara S, et al. Cystoid macular edema associated with topical latanoprost in glaucomatous eyes with a normally functioning blood-ocular barrier. J Glaucoma. 2001;10:233–6.
Yeom HY, Hong S, Kim SS, Kim CY, Seong GJ. Influence of topical bimatoprost on macular thickness and volume in glaucoma patients with phakic eyes. Can J Ophthalmol. 2008;43:563–6.
Miyake K, Ota I, Maekubo K, Ichihashi S, Miyake S. Latanoprost accelerates disruption of the blood-aqueous barrier and the incidence of angiographic cystoid macular edema in early postoperative pseudophakias. Arch Ophthalmol. 1999;117:34–40.
Aihara M, Lu F, Kawata H, Iwata A, Liu K, Odani-Kawabata N, et al. Phase 2, randomized, dose-finding studies of omidenepag isopropyl, a selective EP2 agonist, in patients with primary open-angle glaucoma or ocular hypertension. J Glaucoma. 2019;28:375–85.
Feng Y, Varikooty J, Simpson TL. Diurnal variation of corneal and corneal epithelial thickness measured using optical coherence tomography. Cornea. 2001;20:480–3.
Inoue K. Managing adverse effects of glaucoma medications. Clin Ophthalmol. 2014;8:903–13.
Esaki Y, Katsuta O, Kamio H, Noto T, Mano H, Iwamura R, et al. The antiglaucoma agent and EP2 receptor agonist omidenepag does not affect eyelash growth in mice. J Ocul Pharmacol Ther. 2020;36:529–33.
Yamamoto Y, Taniguchi T, Inazumi T, Iwamura R, Yoneda K, Odani-Kawabata N, et al. Effects of the selective EP2 receptor agonist omidenepag on adipocyte differentiation in 3T3-L1 cells. J Ocul Pharmacol Ther. 2020;36:162–9.
Oogi S, Nakakura S, Terao E, Fujisawa Y, Tabuchi H, Kiuchi Y. One-year follow-up study of changes in prostaglandin-associated periorbital syndrome after switch from conventional prostaglandin F2alfa to omidenepag isopropyl. Cureus. 2020;12: e10064.
Inoue K, Inoue J, Kunimatsu-Sanuki S, Nozaki N, Shimizu K, Ishida K, et al. Short-term efficacy and safety of omidenepag isopropyl in patients with normal-tension glaucoma. Clin Ophthalmol. 2020;14:2943–9.
Acknowledgements
Medical writing was provided by Alex Yardley, BSc, and Jennifer Mitchell, PhD, both of Helios Medical Communications, Cheshire, UK, which was funded by Santen. This study was sponsored by Santen. The authors would like to thank Santen for their support in the development of this manuscript, in particular, Naveed K Shams, MD, PhD, for his input on the study design and for his review of the data presented.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
M. Aihara, Grant, Research support, honoraria/consultation fees, and has participated in a company-sponsored speaker’s bureau (Alcon), Grant, Research support, and has participated in a company-sponsored speaker’s bureau (AMO Japan), Research support (CREWT Medical Systems), Research support, Research support and honoraria/consultation fees (Glaukos), Honoraria/consultation fees (HOYA), Honoraria/consultation fees (IRIDEX), Grant, Research support and has participated in a company-sponsored speaker’s bureau (Kowa Pharmaceutical), Grant, Research support and has participated in a company-sponsored speaker’s bureau (Nippon Tenganyaku), Grant, Research support and has participated in a company-sponsored speaker’s bureau (Novartis), Research support and honoraria/consultation fees (Ono Pharmaceutical), Grant, Research support and has participated in a company-sponsored speaker’s bureau (Otsuka Pharmaceutical), Research support and has participated in a company-sponsored speaker’s bureau (Pfizer), Grant, Research support and honoraria/consultation fees, and has participated in a company-sponsored speaker’s bureau (Santen), Grant (Sato Pharmaceutical), Grant, Research support and honoraria/consultation fees, and has participated in a company-sponsored speaker’s bureau (SENJU Pharmaceutical), Grant, Research support and has participated in a company-sponsored speaker’s bureau (TOMEY), Grant, Research support and honoraria/consultation fees (Wakamoto Pharmaceutical); F. Lu, Employee (Santen); H. Kawata, Employee (Santen); A. Iwata, Employee (Santen); N. Odani-Kawabata, Employee (Santen).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Author: Fenghe Lu
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Aihara, M., Lu, F., Kawata, H. et al. Twelve-month efficacy and safety of omidenepag isopropyl, a selective EP2 agonist, in open-angle glaucoma and ocular hypertension: the RENGE study. Jpn J Ophthalmol 65, 810–819 (2021). https://doi.org/10.1007/s10384-021-00868-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-021-00868-y