Abstract
Purpose
To determine the significance of the correlation between the vascular structure and neural function of the macula in patients with diabetes mellitus.
Study design
Single-center observational study.
Patients and methods
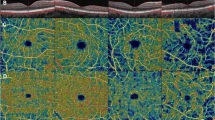
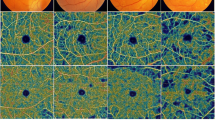
Ninety eyes of 90 diabetic patients with an average (SD) age of 63.5 (3.8) years were studied. Fifty of the eyes had no clinically apparent diabetic retinopathy (non-DR), and 40 eyes had mild-to-moderate nonproliferative DR (NPDR). Thirty age-matched healthy individuals were also studied in the same way. Swept-source optical coherence tomography angiography (OCTA) was performed to obtain 3 × 3-mm en face images of the posterior pole of the eye. The vascular densities (VDs) of the superficial capillary plexus (SCP) and the deep capillary plexus (DCP) were determined. The focal macular electroretinograms (ERGs) elicited by a 15° circular stimulus centered on the fovea were recorded. The amplitudes of the a- and b-waves, sum of the oscillatory potentials (ΣOPs), photopic negative response (PhNR), and implicit times of the individual OPs (OP1-OP3) were measured.
Results
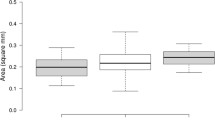
The VDs of the SCP and DCP were reduced in eyes with advanced DR (P < .01 for SCP). The implicit times of OP1-OP3 were significantly prolonged in eyes with a lower VD of the SCP and DCP in the non-DR group (P < .05). The amplitudes of the ΣOPs were significantly smaller in eyes with a reduced VD of the SCP and DCP in the NPDR group (P < .05). The correlation coefficients were higher for the OP implicit times than for the ΣOP amplitudes in the non-DR group.
Conclusions
The OPs of the focal macular ERG are smaller with prolonged implicit times in association with capillary loss in the macula of diabetic patients. The implicit times are the most sensitive functional parameter that reflects the early changes of the microvasculature in the macula caused by diabetes.








Similar content being viewed by others
References
Wong TY, Cheung CMG, Larsen M, Sharma S, Simó R. Diabetic retinopathy. Nat Rev Dis Primers. 2016;2:16012.
Yonemura D, Aoki T, Tsuzuki K. Electroretinogram in diabetic retinopathy. Arch Ophthalmol. 1962;68:19–24.
Shirao Y, Kawasaki K. Electrical responses from diabetic retina. Prog Retin Eye Res. 1998;17:59–76.
Yonemura D, Tsuzuki K, Aoki T. Clinical importance of the oscillatory potential in the human ERG. Acta Ophthalmol. 1962;Suppl 70:115–23.
Wachtmeister L. Oscillatory potentials in the retina: what do they reveal. Prog Retin Eye Res. 1998;17:485–521.
Kawasaki K. Preretinopathic changes in the oscillatory potential in diabetic retina: interpretation and significance. Article in Japanese. Nippon Ganka Gakkai Zasshi. 1998;102:813–36.
Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical coherence tomography angiography. Prog Retin Eye Res. 2018;64:1–55.
Takase N, Nozaki M, Kato A, Ozeki H, Yoshida M, Ogura Y. Enlargement of foveal avascular zone in diabetic eyes evaluated by en face optical coherence tomography angiography. Retina. 2015;35:2377–83.
Ishibazawa A, Nagaoka T, Takahashi A, Omae T, Tani T, Sogawa K, et al. Optical coherence tomography angiography in diabetic retinopathy: a prospective pilot study. Am J Ophthalmol. 2015;160:35–44.
Salz DA, de Carlo TE, Adhi M, Moult E, Choi W, Baumal CR, et al. Select features of diabetic retinopathy on swept-source optical coherence tomographic angiography compared with fluorescein angiography and normal eyes. JAMA Ophthalmol. 2016;34:644–50.
Couturier A, Rey P-A, Erginay A, Lavia C, Bonnin S, Dupas B, et al. Widefield OCT-angiography and fluorescein angiography assessments of nonperfusion in diabetic retinopathy and edema treated with anti-vascular endothelial growth factor. Ophthalmology. 2019;126:1685–94.
Miyake Y, Yanagida K, Kondo K, Ota I. Subjective scotometry and recording of local electroretinogram and visual evoked response: system with television monitor of the fundus. Jpn J Ophthalmol. 1981;25:439–48.
Miyake Y. Studies on local macular ERG. Acta Soc Ophthalmol Jpn. 1988;92:1419–49.
Miyake Y. Macular oscillatory potentials in humans: macular OPs. Doc Ophthalmol. 1990;75:111–24.
Miyake Y, Shiroyama N, Horiguchi M, Ota I. Asymmetry of focal ERG in human macular region. Invest Ophthalmol Vis Sci. 1989;30:1743–9.
Yoon IH, Shiroyama N, Miyake Y, Awaya S. Oscillatory potentials of local macular ERG in diabetic retinopathy. Korean J Ophthalmol. 1990;4:40–5.
Wilkinson CP, Ferris FL, Klein RE, Lee PP, Agardh CD, Davis M, et al. Proposed international diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–82.
Sawada O, Ichiyama Y, Obata S, Ito Y, Kakinoki M, Sawada T, et al. Comparison between wide-angle OCT angiography and ultra-wide field fluorescein angiography for detecting non-perfusion areas and retinal neovascularization in eyes with diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2018;256:1275–80.
Hirano T, Kakihara S, Toriyama Y, Nittala MG, Murata T, Sadda S. Wide-field en face swept-source optical coherence tomography angiography using extended field imaging in diabetic retinopathy. Br J Ophthalmol. 2018;102:1199–203.
Zeng Y, Cao D, Yu H, Yang D, Zhuang X, Hu Y, et al. Early retinal neurovascular impairment in patients with diabetes without clinically detectable retinopathy. Br J Ophthalmol. 2019;103:1747–52.
Zeng Y, Cao D, Yang D, Zhuang X, Hu Y, He M, et al. Retinal vasculature-function correlation in non-proliferative diabetic retinopathy. Doc Ophthalmol. 2020;140:129–38.
Kondo M, Sieving PA. Primate photopic sine-wave flicker ERG: vector modeling analysis of component origins using glutamate analogs. Invest Ophthalmol Vis Sci. 2001;42:305–12.
Kondo M, Sieving PA. Post-photoreceptoral activity dominates primate photopic 32-Hz ERG for sine-, square-, and pulsed stimuli. Invest Ophthalmol Vis Sci. 2002;43:2500–7.
Park JC, Chen YF, Liu M, Liu K, McAnany JJ. Structural and functional abnormalities in early-stage diabetic retinopathy. Curr Eye Res. 2020. https://doi.org/10.1080/02713683.
Vujosevic S, Muraca A, Gatti V, Masoero L, Brambilla M, Cannillo B, et al. Peripapillary microvascular and neural changes in diabetes mellitus: an OCT-angiography study. Invest Ophthalmol Vis Sci. 2018;59:5074–81.
Viswanathan S, Frishman LJ, Robson JG, Harwerth RS, Smith EL 3rd. The photopic negative response of the macaque electroretinogram: reduction by experimental glaucoma. Invest Ophthalmol Vis Sci. 1999;40:1124–36.
Viswanathan S, Frishman LJ, Robson JG, Walter JW. The photopic negative response of the flash electroretinogram in primary open angle glaucoma. Invest Ophthalmol Vis Sci. 2001;42:514–22.
Gotoh Y, Machida S, Tazawa Y. Selective loss of the photopic negative response in patients with optic nerve atrophy. Arch Ophthalmol. 2004;122:341–6.
Machida S, Gotoh Y, Toba Y, Ohtaki A, Kaneko M, Kurosaka D. Correlation between photopic negative response and retinal nerve fiber layer thickness and optic disc topography in glaucomatous eyes. Invest Ophthalmol Vis Sci. 2008;49:2201–7.
Machida S. Clinical application of the photopic negative response to optic nerve and retinal diseases. J Ophthalmol. 2012. https://doi.org/10.1155/397178.
Kondo M, Kurimoto Y, Sakai T, Koyasu T, Miyata K, Ueno S, et al. Recording focal macular photopic negative response (PhNR) from monkeys. Invest Ophthalmol Vis Sci. 2008;49:3544–50.
Machida S, Toba Y, Ohtaki A, Gotoh Y, Kaneko M, Kurosaka D. Photopic negative response of focal electoretinograms in glaucomatous eyes. Invest Ophthalmol Vis Sci. 2008;49:5636–44.
Machida S, Tamada K, Oikawa T, Yokoyama D, Kaneko M, Kurosaka D. Sensitivity and specificity of photopic negative response of focal electroretinogram to detect glaucomatous eyes. Br J Ophthalmol. 2010;94:202–8.
Tamada K, Machida S, Yokoyama D, Kurosaka D. Photopic negative response of full-field and focal electroretinograms in patients with optic nerve atrophy. Jpn J Ophthalmol. 2009;53:608–14.
Tamada K, Machida S, Oikawa T, Miyamoto H, Nishimura T, Kurosaka D. Correlation between photopic negative response of focal electroretinograms and local loss of retinal neurons in glaucoma. Curr Eye Res. 2010;35:155–64.
Kizawa J, Machida S, Kobayashi T, Gotoh Y, Kurosaka D. Changes of oscillatory potentials and photopic negative response in patients with early diabetic retinopathy. Jpn J Ophthalmol. 2006;50:367–73.
Siliprandi R, Canella R, Carmignoto G, Schiavo N, Zanellato A, Zanoni R, et al. N-methyl-D-aspartate-induced neurotoxicity in the adult rat retina. Vis Neurosci. 1992;8:567–73.
Spix NJ, Liu LL, Zhang Z, Hohlbein JP, Prigge CL, Chintala S, et al. Vulnerability of dopaminergic amacrine cells to chronic ischemia in a mouse model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2016;57:3047–57.
Luu CD, Szental JA, Lee S, Lavanya R, Wong TY. Correlation between retinal oscillatory potentials and retinal vascular caliber in type 2 diabetes. Invest Ophthalmol Vis Sci. 2010;51:482–6.
Funding
This research was supported by Japan Society for the Promotion of Science (Grant 18K09420).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
S. Ebihara, None; S. Machida, None; Y. Hara, None; A. Tada, None; M. Ishizuka, None; M. Gonmori, None; T. Nishimura, None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Author: Shigeki Machida
About this article
Cite this article
Ebihara, S., Machida, S., Hara, Y. et al. Relationships between the vascular structure and neural function of the macula in patients with diabetes mellitus. Jpn J Ophthalmol 65, 77–88 (2021). https://doi.org/10.1007/s10384-020-00784-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-020-00784-7