Abstract
Purpose
To determine the presence or absence of interocular differences in the foveal avascular zone (FAZ) area in healthy eyes.
Study design
Cross-sectional study.
Methods
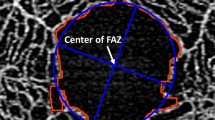
We examined 236 healthy eyes of 118 consecutive subjects (mean age, 39.1 ± 18.9 years). We used swept-source optical coherence tomography angiography (OCTA) images of the FAZ to measure its area from both the superficial capillary plexus (SCP-FAZ) and the whole retinal capillary plexus (WCP-FAZ). We also investigated the relationship between interocular differences in SCP-FAZ and other factors such as: axial length, spherical equivalent, central retinal thickness, and retinal vascular density.
Results
There was no significant difference in the FAZ area between the right and left eyes in either the SCP-FAZ (P = 0.61) or WCP-FAZ (P = 0.80), and the FAZ areas of both eyes showed significant positive correlations (SCP-FAZ; P < 0.001, R2 = 0.884, WCP-FAZ; P < 0.001, R2 = 0.856). Bland–Altman plots showed that the mean interocular difference in SCP-FAZ area was 0.002 ± 0.037 mm2 (95% confidence interval, -0.072–0.075 mm2), and in the WCP-FAZ area, 0.050 ± 0.044 mm2 (95% confidence interval, -0.036–0.137 mm2). Multivariate regression analysis showed that none of the investigated factors were significantly associated with interocular differences in SCP-FAZ (P = 0.61, R2 = 0.138).
Conclusions
There was no significant interocular difference in SCP- and WCP-FAZ areas in healthy eyes. The normal range of values for interocular difference in SCP-FAZ area was 0.002 ± 0.037 mm2 and in the WCP-FAZ area, 0.050 ± 0.044 mm2.




Similar content being viewed by others
References
Spaide RF, Klancnik JM, Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015;133:45–50.
Samara WA, Say EAT, Khoo CTL, Higgins TP, Magrath G, Ferenczy S, et al. Correlation of foveal avascular zone size with foveal morphology in normal eyes using optical coherence tomography angiography. Retina. 2015;35:2188–95.
Lupidi M, Coscas F, Cagini C, Fiore T, Spaccini E, Fruttini D, et al. Automated quantitative analysis of retinal microvasculature in normal eyes on optical coherence tomography angiography. Am J Ophthalmol. 2016;169:9–23.
Wylęgała A, Teper S, Dobrowolski D, Wylęgała E. Optical coherence angiography: a review. Medicine. 2016;95:e4907.
Gadde SGK, Anegondi N, Bhanushali D, Chidambara L, Yadav NK, Khurana A, et al. Quantification of vessel density in retinal optical coherence tomography angiography images using local fractal dimension. Invest Ophthalmol Vis Sci. 2016;57:246–52.
Ghasemi Falavarjani K, Iafe NA, Hubschman J-P, Tsui I, Sadda SR, Sarraf D. Optical coherence tomography angiography analysis of the foveal avascular zone and macular vessel density after anti-VEGF therapy in eyes with diabetic macular edema and retinal vein occlusion. Invest Ophthalmol Vis Sci. 2017;58:30–4.
Balaratnasingam C, Inoue M, Ahn S, McCann J, Dhrami-Gavazi E, Yannuzzi LA, et al. Visual acuity is correlated with the area of the foveal avascular zone in diabetic retinopathy and retinal vein occlusion. Ophthalmology. 2016;123:2352–67.
Bhanushali D, Anegondi N, Gadde SGK, Srinivasan P, Chidambara L, Yadav NK, et al. Linking retinal microvasculature features with severity of diabetic retinopathy using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57:OCT519–25.
Samara WA, Shahlaee A, Adam MK, Khan MA, Chiang A, Maguire JI, et al. Quantification of diabetic macular ischemia using optical coherence tomography angiography and its relationship with visual acuity. Ophthalmology. 2017;124:235–44.
Samara WA, Shahlaee A, Sridhar J, Khan MA, Ho AC, Hsu J. Quantitative optical coherence tomography angiography features and visual function in eyes with branch retinal vein occlusion. Am J Ophthalmol. 2016;166:76–83.
Adhi M, Filho MAB, Louzada RN, Kuehlewein L, de Carlo TE, Baumal CR, et al. Retinal capillary network and foveal avascular zone in eyes with vein occlusion and fellow eyes analyzed with optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57:OCT486–94.
Suzuki N, Hirano Y, Tomiyasu T, Esaki Y, Uemura A, Yasukawa T, et al. Retinal hemodynamics seen on optical coherence tomography angiography before and after treatment of retinal vein occlusion. Invest Ophthalmol Vis Sci. 2016;57:5681–7.
Tan CS, Lim LW, Chow VS, Chay IW, Tan S, Cheong KX, et al. Optical coherence tomography angiography evaluation of the parafoveal vasculature and its relationship with ocular factors. Invest Ophthalmol Vis Sci. 2016;57:OCT224–34.
Shahlaee A, Pefkianaki M, Hsu J, Ho AC. Measurement of foveal avascular zone dimensions and its reliability in healthy eyes using optical coherence tomography angiography. Am J Ophthalmol. 2016;161:50–5.
Yu J, Jiang C, Wang X, Zhu L, Gu R, Xu H, et al. Macular perfusion in healthy Chinese: an optical coherence tomography angiogram study. Invest Ophthalmol Vis Sci. 2015;56:3212–7.
Kuehlewein L, Tepelus TC, An L, Durbin MK, Srinivas S, Sadda SR. Noninvasive visualization and analysis of the human parafoveal capillary network using swept source OCT optical microangiography. Invest Ophthalmol Vis Sci. 2015;56:3984–8.
Fujiwara A, Morizane Y, Hosokawa M, Kimura S, Shiode Y, Hirano M, et al. Factors affecting foveal avascular zone in healthy eyes: An examination using swept-source optical coherence tomography angiography. PLoS One. 2017;12:e0188572.
Yasuno Y, Miura M, Kawana K, Makita S, Sato M, Okamoto F, et al. Visualization of sub-retinal pigment epithelium morphologies of exudative macular diseases by high-penetration optical coherence tomography. Invest Ophthalmol Vis Sci. 2009;50:405–13.
Fujiwara A, Morizane Y, Hosokawa M, Kimura S, Kumase F, Shiode Y, et al. Factors affecting choroidal vascular density in normal eyes: quantification using en face swept-source optical coherence tomography. Am J Ophthalmol. 2016;170:1–9.
Al-Sheikh M, Akil H, Pfau M, Sadda SR. Swept-source OCT angiography imaging of the foveal avascular zone and macular capillary network density in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2016;57:3907–13.
Yoshikawa Y, Shoji T, Kanno J, Ibuki H, Ozaki K, Ishii H, et al. Evaluation of microvascular changes in the macular area of eyes with rhegmatogenous retinal detachment without macular involvement using swept-source optical coherence tomography angiography. Clin Ophthalmol. 2018;12:2059–67.
Sonoda S, Sakamoto T, Yamashita T, Uchino E, Kawano H, Yoshihara N, et al. Luminal and stromal areas of choroid determined by binarization method of optical coherence tomographic images. Am J Ophthalmol. 2015;159:1123–31.
Sonoda S, Sakamoto T, Yamashita T, Shirasawa M, Uchino E, Terasaki H, et al. Choroidal structure in normal eyes and after photodynamic therapy determined by binarization of optical coherence tomographic images. Invest Ophthalmol Vis Sci. 2014;55:3893–9.
Tan CS, Chan JC, Cheong KX, Ngo WK, Sadda SR. Comparison of retinal thicknesses measured using swept-source and spectral-domain optical coherence tomography devices. Ophthalmic Surg Lasers Imaging Retina. 2015;46:172–9.
Michalewska Z, Michalewski J, Ornafel-Sagan K, Nawrocki J. Swept-source optical coherence tomography correlations between retina and choroid before and after vitrectomy for epiretinal membranes. Am J Ophthalmol. 2016;165:100–7.
Rispoli M, Savastano MC, Lumbroso B. Capillary network anomalies in branch retinal vein occlusion on optical coherence tomography angiography. Retina. 2015;35:2332–8.
Wakabayashi T, Sato T, Hara-Ueno C, Fukushima Y, Sayanagi K, Shiraki N, et al. Retinal microvasculature and visual acuity in eyes with branch retinal vein occlusion: imaging analysis by optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2017;58:2087–94.
Mammo Z, Balaratnasingam C, Yu P, Xu J, Heisler M, Mackenzie P, et al. Quantitative noninvasive angiography of the fovea centralis using speckle variance optical coherence tomography. Invest Ophthalmol Vis Sci. 2015;56:5074–86.
Liu G, Keyal K, Wang F. Interocular symmetry of vascular density and association with central macular thickness of healthy adults by optical coherence tomography angiography. Sci Rep. 2017;7:16297.
Shahlaee A, Samara WA, Hsu J, Say EAT, Khan MA, Sridhar J, et al. In vivo assessment of macular vascular density in healthy human eyes using optical coherence tomography angiography. Am J Ophthalmol. 2016;165:39–46.
Spaide RF, Fujimoto JG, Waheed NK. Image artifacts in optical coherence tomography angiography. Retina. 2015;35:2163–80.
Spaide RF, Curcio CA. Evaluation of segmentation of the superficial and deep vascular layers of the retina by optical coherence tomography angiography instruments in normal eyes. JAMA Ophthalmol. 2017;135:259–62.
Takase N, Nozaki M, Kato A, Ozeki H, Yoshida M, Ogura Y. Enlargement of foveal avascular zone in diabetic eyes evaluated by en face optical coherence tomography angiography. Retina. 2015;35:2377–83.
Iafe NA, Phasukkijwatana N, Chen X, Sarraf D. Retinal capillary density and foveal avascular zone area are age-dependent: quantitative analysis using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57:5780–7.
Girkin CA, McGwin G, Sinai MJ, Sekhar GC, Fingeret M, Wollstein G, et al. Variation in optic nerve and macular structure with age and race with spectral-domain optical coherence tomography. Ophthalmology. 2011;118:2403–8.
Knight OJ, Girkin CA, Budenz DL, Durbin MK, Feuer WJ. Effect of race, age, and axial length on optic nerve head parameters and retinal nerve fiber layer thickness measured by cirrus HD-OCT. Arch Ophthalmol. 2012;130:312–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
M. Liu, None; A. Fujiwara, None; Y. Morizane, None; R. Kawasaki, None; S. Kimura, None; M. Morizane-Hosokawa, None; Y. Shiode, None; M. Hirano, None; S. Doi, None; S. Toshima, None; K. Takahashi, None; M. Hosogi, None; X. Ma, None; F. Shiraga, None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Author: Yuki Morizane
Electronic supplementary material
Below is the link to the electronic supplementary material.
10384_2020_719_MOESM1_ESM.tif
Supplementary Fig. 1 Interocular differences in axial length and spherical equivalent. (a and b) axial length showed a strong positive correlation between fellow eyes (P < 0.001, R2 = 0.956), and the interocular difference was not significant (P = 0.44). (c and d) Spherical equivalent showed a strong positive correlation between fellow eyes (P < 0.001, R2 = 0.954), and the interocular difference was not significant (P = 0.25) (TIFF 310 kb)
10384_2020_719_MOESM2_ESM.tif
Supplementary Fig. 2 Interocular differences in central retinal thickness and retinal vascular density. (a and b) central retinal thickness showed a strong positive correlation between fellow eyes (P < 0.001, R2 = 0.745), and the interocular difference was not significant (P = 0.35). (c and d) Retinal vascular density showed a strong positive correlation between fellow eyes (P < 0.001, R2 = 0.962), and the interocular difference was not significant (P = 0.41) (TIFF 328 kb)
About this article
Cite this article
Liu, M., Fujiwara, A., Morizane, Y. et al. Interocular symmetry of the foveal avascular zone area in healthy eyes: a swept-source optical coherence tomography angiography study. Jpn J Ophthalmol 64, 171–179 (2020). https://doi.org/10.1007/s10384-020-00719-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-020-00719-2