Abstract
The Retinal Function Imager (RFI; Optical Imaging, Rehovot, Israel) is a unique, noninvasive multiparameter functional imaging instrument that directly measures hemodynamic parameters such as retinal blood-flow velocity, oximetric state, and metabolic responses to photic activation. In addition, it allows capillary perfusion mapping without any contrast agent. These parameters of retinal function are degraded by retinal abnormalities. This review delineates the development of these parameters and demonstrates their clinical applicability for noninvasive detection of retinal function in several modalities. The results suggest multiple clinical applications for early diagnosis of retinal diseases and possible critical guidance of their treatment.
Similar content being viewed by others
References
Fujimoto JG. Optical coherence tomography for ultrahigh resolution in vivo imaging. Nat Biotechnol 2003;21:1361–1367.
Trick GL, Calotti FY, Skarf B. Advances in imaging of the optic disc and retinal nerve fiber layer. J Neuroophthalmol 2006;26:284–295.
Singh R, Kaiser PK. Advances in AMD imaging. Int Ophthalmol Clin 2007;47:65–74.
Drexler W, Fujimoto JG. State-of-the-art retinal optical coherence tomography. Prog Retin Eye Res 2008;27:45–88.
Schmitz-Valckenberg S, Holz FG, Bird AC, Spaide RF. Fundus autofluorescence imaging: review and perspectives. Retina 2008;28:385–409.
Podoleanu AG, Rosen RB. Combinations of techniques in imaging the retina with high resolution. Prog Retin Eye Res 2008;27:464–499.
Grinvald A, Bonhoeffer T, Vanzetta I, et al. High-resolution functional optical imaging: from the neocortex to the eye. Ophthalmol Clin North Am 2004;17:53–67.
Nelson DA, Krupsky S, Pollack A, et al. Special report: noninvasive multi-parameter functional optical imaging of the eye. Ophthalmic Surg Lasers Imaging 2005;36:57–66.
Denninghoff KR, Smith MH, Hillman L. Retinal imaging techniques in diabetes. Diabetes Technol Ther 2000;2:111–113.
Harris A, Dinn RB, Kagemann L, Rechtman E. A review of methods for human retinal oximetry. Ophthalmic Surg Lasers Imaging 2003;34:152–164.
Blum M, Bachmann K, Wintzer D, Riemer T, Vilser W, Strobel J. Noninvasive measurement of the Bayliss effect in retinal autoregulation. Graefes Arch Clin Exp Ophthalmol 1999;237:296–300.
Hubbard LD, Brothers RJ, King WN, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology 1999;106:2269–2280.
Ege BM, Hejlesen OK, Larsen OV, et al. Screening for diabetic retinopathy using computer based image analysis and statistical classification. Comput Methods Programs Biomed 2000;62:165–175.
van Hecke MV, Dekker JM, Nijpels G, et al. Are retinal microvascular abnormalities associated with large artery endothelial dysfunction and intima-media thickness? The Hoorn Study. Clin Sci (Lond) 2006;110:597–604.
Stanton AV, Wasan B, Cerutti A, et al. Vascular network changes in the retina with age and hypertension. J Hypertens 1995;13(12 Pt 2):1724–1728.
van den Born BJ, Hulsman CA, Hoekstra JB, Schlingemann RO, van Montfrans GA. Value of routine funduscopy in patients with hypertension: systematic review. BMJ 2005;331:73.
Landa G, Garcia PM, Rosen RB. Correlation between retina blood flow velocity assessed by retinal function imager and retina thickness estimated by scanning laser ophthalmoscopy/optical coherence tomography. Ophthalmologica 2009;223:155–161.
Kwan AS, Barry C, McAllister IL, Constable I. Fluorescein angiography and adverse drug reactions revisited: the Lions Eye experience. Clin Exp Ophthalmol 2006;34:33–38.
Stefansson E, Landers MB 3rd, Wolbarsht ML. Oxygenation and vasodilatation in relation to diabetic and other proliferative retinopathies. Ophthalmic Surg 1983;14:209–226.
Stefansson E, Machemer R, de Juan E Jr, McCuen BW 2nd, Peterson J. Retinal oxygenation and laser treatment in patients with diabetic retinopathy. Am J Ophthalmol 1992;113:36–38.
Tiedeman JS, Kirk SE, Srinivas S, Beach JM. Retinal oxygen consumption during hyperglycemia in patients with diabetes without retinopathy. Ophthalmology 1998;105:31–36.
Sebag J, Delori FC, Feke GT, Weiter JJ. Effects of optic atrophy on retinal blood flow and oxygen saturation in humans. Arch Ophthalmol 1989;107:222–226.
Grinvald A, Lieke E, Frostig RD, Gilbert CD, Wiesel TN. Functional architecture of cortex revealed by optical imaging of intrinsic signals. Nature 1986;324:361–364.
Grinvald A, Sharon D, Vanzetta I, Slovin H. Intrinsic signal imaging in the neocortex. In: Yuste R, Konnerth A, editors. Imaging in neuroscience and development: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2005. p. xvi.
Grinvald A, Shoam D, Shmuel DE, et al. In-vivo optical imaging of cortical architecture and dynamics. Modern techniques in neuroscience research. Heidelberg: Springer; 1999.
Hill DK, Keynes RD. Opacity changes in stimulated nerve. J Physiol 1949;108:278–281.
Cohen LB, Keynes RD, Hille B. Light scattering and birefringence changes during nerve activity. Nature 1968;218:438–441.
Cohen LB. Changes in neuron structure during action potential propagation and synaptic transmission. Physiol Rev 1973;53:373–418.
Frostig RD, Lieke EE, Ts’o DY, Grinvald A. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc Natl Acad Sci U S A 1990;87:6082–6086.
Malonek D, Grinvald A. Interactions between electrical activity and cortical microcirculation revealed by imaging spectroscopy: implications for functional brain mapping. Science 1996;272:551–554.
Tsunoda K, Oguchi Y, Hanazono G, Tanifuji M. Mapping cone- and rod-induced retinal responsiveness in macaque retina by optical imaging. Invest Ophthalmol Vis Sci 2004;45:3820–3826.
Abramoff MD, Kwon YH, Ts’o D, et al. Visual stimulus-induced changes in human near-infrared fundus reflectance. Invest Ophthalmol Vis Sci 2006;47:715–721.
Hanazono G, Tsunoda K, Shinoda K, Tsubota K, Miyake Y, Tanifuji M. Intrinsic signal imaging in macaque retina reveals different types of flash-induced light reflectance changes of different origins. Invest Ophthalmol Vis Sci 2007;48:2903–2912.
Hanazono G, Tsunoda K, Kazato Y, Tsubota K, Tanifuji M. Evaluating neural activity of retinal ganglion cells by flash-evoked intrinsic signal imaging in macaque retina. Invest Ophthalmol Vis Sci 2008;49:4655–4663.
Grieve K, Roorda A. Intrinsic signals from human cone photoreceptors. Invest Ophthalmol Vis Sci 2008;49:713–719.
Srinivasan VJ, Chen Y, Duker JS, Fujimoto JG. In vivo functional imaging of intrinsic scattering changes in the human retina with high-speed ultrahigh resolution OCT. Opt Exp 2009;17:3861–3877.
Author information
Authors and Affiliations
Corresponding author
Additional information
Electronic supplementary material The online version of this article (doi: 10.1007/s10384-009-0689-0) contains supplementary material, which is available to authorized users.
Electronic supplementary material
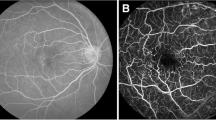
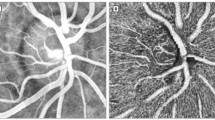
Flow from a normal subject
The re-registration and differential processing of a series of images taken at 50–60 Hz produces a “flow movie,” in which it is possible to follow the motion of individual clusters of red blood cells. The movie linked to here is derived from a series of eight aligned images obtained over a period of 100 ms. Dividing each single image by the average of all produces eight corresponding differential images; played in order, these comprise the flow movie itself. Interpretation of a flow movie is straightforward: where there is a black spot, there is an erythrocyte or erythrocyte cluster; in white spots, or gaps, erythrocytes are absent.
The movie is of the same region depicted in Fig. 3B in this article.
About this article
Cite this article
Izhaky, D., Nelson, D.A., Burgansky-Eliash, Z. et al. Functional imaging using the retinal function imager: Direct imaging of blood velocity, achieving fluorescein angiography-like images without any contrast agent, qualitative oximetry, and functional metabolic signals. Jpn J Ophthalmol 53, 345–351 (2009). https://doi.org/10.1007/s10384-009-0689-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-009-0689-0