Summary
Background
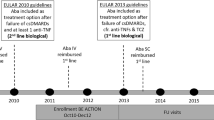
AbataCepT In rOutiNe clinical practice (ACTION; NCT02109666) was a 2-year international observational study of patients with moderate to severe rheumatoid arthritis.
Methods
Baseline characteristics, abatacept retention rates, and clinical outcomes were compared by treatment line in the Austrian cohort of ACTION.
Results
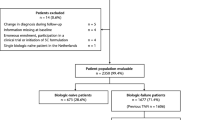
Of 100 patients enrolled in Austria, 98 (98.0%) were evaluable: 33/98 (33.7%) biologic naïve and 65/98 (66.3%) with ≥1 prior biologic failure. At baseline, biologic-naïve patients had shorter disease duration and lower concomitant corticosteroid use than biologic-failure patients. Overall crude abatacept retention rate was 60.5% and retention rate was higher in biologic-naïve (65.1%) versus biologic-failure (58.0%) patients. Good/moderate EULAR (European League Against Rheumatism) response rates were 85.7% in biologic-naïve and 100% in biologic-failure patients.
Conclusions
In the Austrian cohort of ACTION, overall abatacept retention at 2 years was high, with higher retention rates in patients receiving abatacept as an earlier treatment line. Good/moderate EULAR response rate was higher in biologic-failure than in biologic-naïve patients.
Zusammenfassung
Grundlagen
„AbataCepT In rOutiNe clinical practice“ (ACTION; NCT02109666) war eine internationale 2‑Jahres-Beobachtungsstudie an Patienten mit mittel- bis schwergradiger rheumatoider Arthritis.
Methodik
Ausgangsdaten, Raten der Abatacepttherapietreue und klinische Ergebnisse wurden in Hinsicht auf die Therapielinie in der österreichischen Kohorte der ACTION-Studie verglichen.
Ergebnisse
Von 100 Patienten, die in Österreich in die Studie aufgenommen wurden, waren die Daten von 98 (98,0%) auswertbar: Biologikanaiv waren 33/98 (33,7%) und bei 65/98 (66,3%) lag mindestens ein vorheriges Biologikatherapieversagen vor. Zu Studienbeginn wiesen die biologikanaiven Patienten eine kürzere Krankheitsdauer und einen geringeren Einsatz von Kortikosteroiden als Begleitmedikation auf als die Patienten mit Biologikatherapieversagen. Die rohe Gesamttherapietreuerate zu Abatacept betrug 60,5%, und die Therapietreuerate war bei biologikanaiven Patienten (65,1%) höher als bei Patienten mit Biologikatherapieversagen (58,0%). Gute/mittelmäßige Therapieansprechraten gemäß EULAR-Kriterien (European League Against Rheumatism) gab es bei 85,7% der biologikanaiven Patienten und bei 100% derer mit Biologikatherapieversagen.
Schlussfolgerungen
In der österreichischen Kohorte der ACTION-Studie war die Gesamttherapietreue zu Abatacept nach 2 Jahren hoch, dabei bestanden höhere Therapietreueraten bei Patienten, die Abatacept eher als Therapie erhielten. Eine gute/mittelmäßige Ansprechrate gab es in höherem Maße bei Patienten mit Biologikatherapieversagen als bei biologikanaiven Patienten.




Similar content being viewed by others
References
Smolen JS, Landewé R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960–77.
Cross M, Smith E, Hoy D, Carmona L, Wolfe F, Vos T, et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1316–22.
European Commission. The 2018 ageing report: economic and budgetary projections for the EU member states (2016–2070). 2018.
Smolen JS, Landewe R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73(3):492–509.
Smolen JS. Treat-to-target as an approach in inflammatory arthritis. Curr Opin Rheumatol. 2016;28(3):297–302.
Du Pan SM, Dehler S, Ciurea A, Ziswiler HR, Gabay C, Finckh A. Comparison of drug retention rates and causes of drug discontinuation between anti-tumor necrosis factor agents in rheumatoid arthritis. Arthritis Rheumatol. 2009;61(5):560–8.
Ebina K, Hashimoto M, Yamamoto W, Ohnishi A, Kabata D, Hirano T, et al. Drug retention and discontinuation reasons between seven biologics in patients with rheumatoid arthritis—the ANSWER cohort study. PLoS ONE. 2018;13(3):e0194130. https://doi.org/10.1371/journal.pone0194130.
electronic medicines compendium. Orencia 250 mg powder for concentrate for solution for infusion. 2017. http://www.medicines.org.uk/emc/medicine/19714/SPC/. Accessed 01 May 2019.
Alten R, Mariette X, Lorenz HM, Galeazzi M, Cantagrel A, Nusslein HG, et al. Real-world predictors of 12-month intravenous abatacept retention in patients with rheumatoid arthritis in the ACTION observational study. RMD Open. 2017;3(2):e538.
Nüßlein H, Alten R, Galeazzi M, Lorenz HM, Boumpas D, Nurmohamed MT, et al. Real-world effectiveness of abatacept for rheumatoid arthritis treatment in European and Canadian populations: a 6-month interim analysis of the 2‑year, observational, prospective ACTION study. BMC Musculoskelet Disord. 2014;15(1):14.
Nüßlein HG, Alten R, Galeazzi M, Lorenz HM, Nurmohamed MT, Bensen WG, et al. Efficacy and prognostic factors of treatment retention with intravenous abatacept for rheumatoid arthritis: 24-month results from an international, prospective, real-world study. Clin Exp Rheumatol. 2016;34(3):489–99.
Prodinger B, Ndosi M, Nordenskiold U, Stamm T, Persson G, Andreasson I, et al. Rehabilitation provided to patients with rheumatoid arthritis: a comparison of three different rheumatology clinics in Austria, Sweden and the UK from the perspectives of patients and health professionals. J Rehabil Med. 2015;47(2):174–82.
Puchner R, Hochreiter R, Pieringer H, Vavrovsky A. Improving patient flow of people with rheumatoid arthritis has the potential to simultaneously improve health outcomes and reduce direct costs. BMC Musculoskelet Disord. 2017;18(1):7.
Alten R, Mariette X, Nuesslein H, Galeazzi M, Navarro F, Chartier M, et al. Abatacept retention rates and prognostic factors of retention in patients with rheumatoid arthritis: 2‑year results from the real-world ACTION study. Ann Rheum Dis. 2017;76(Suppl 2):FRI245.
Alten R, Lorenz HM, Mariette X, Nüßlein H, Galeazzi M, Navarro F, et al. Abatacept retention rates, overall and by participating country, and prognostic factors of retention in patients with RA: 2‑year results from a real-world observational study. In: ACR/ARHP Annual Scientific Meeting, San Diego, CA, USA, November 4–8. 2017. p. Poster 1468.
Bristol-Myers Squibb. Orencia (abatacept) summary of product characteristics. 2017. http://www.medicines.org.uk/emc/medicine/27216/SPC/ORENCIA+125+mg+solution+for+injection+(pre-filled+syringe). Accessed 17 Apr 2018.
Canada B‑MS. Orencia (abatacept) product monograph. 2017. https://www.bms.com/assets/bms/ca/documents/productmonograph/ORENCIA_EN_PM.pdf. Accessed 2 Feb 2018.
World Medical Association Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. J Am Med Assoc. 1997;277(11):925–6.
ICH Harmonised Tripartite Guideline. Guideline for good clinical practice. J Postgrad Med. 2001;47(3):199–203.
International Epidemiological Association. Good epidemiological practice (GEP): IEA guidelines for proper conduct in epidemiologic research: IEA-European Federation. 2007. http://ieaweb.org/good-epidemiological-practice-gep/. Accessed May 2007.
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheumatol. 1988;31(3):315–24.
Fransen J, van Riel PL. The Disease Activity Score and the EULAR response criteria. Rheum Dis Clin North Am. 2009;35(4):745–57.
Felson DT, Smolen JS, Wells G, Zhang B, van Tuyl LH, Funovits J, et al. American College of Rheumatology/European League against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Ann Rheum Dis. 2011;70(3):404–13.
Stamm TA, Reichardt B, Zwerina J, Ritschl V, Nell-Duxneuner V. Use of biological disease modifying antirheumatic drugs in rheumatoid arthritis in Austria from 2008 to 2011: a retrospective analysis of 72 % of the population. Wien Klin Wochenschr. 2018;130(7–8):230–7.
Alten R, Nusslein HG, Mariette X, Galeazzi M, Lorenz HM, Cantagrel A, et al. Baseline autoantibodies preferentially impact abatacept efficacy in patients with rheumatoid arthritis who are biologic naive: 6‑month results from a real-world, international, prospective study. RMD Open. 2017;3(1):e345. https://doi.org/10.1136/rmdopen-2016-000345.
WHO. World Health Organization global database. 2018. http://www.who.int/countries/en/. Accessed 3.8.
van der Zee-Neuen A, Putrik P, Ramiro S, Keszei AP, Hmamouchi I, Dougados M, et al. Large country differences in work outcomes in patients with RA—an analysis in the multinational study COMORA. Arthritis Res Ther. 2017. https://doi.org/10.1186/s13075-017-1421-y.
Hifinger M, Hiligsmann M, Ramiro S, Watson V, Severens JL, Fautrel B, et al. Economic considerations and patients’ preferences affect treatment selection for patients with rheumatoid arthritis: a discrete choice experiment among European rheumatologists. Ann Rheum Dis. 2017;76(1):126–32.
Albrecht K, Krüger K, Wollenhaupt J, Alten R, Backhaus M, Baerwald C, et al. German guidelines for the sequential medical treatment of rheumatoid arthritis with traditional and biologic disease-modifying antirheumatic drugs. Rheumatol Int. 2014;34(1):1–9.
Emery P, Solem C, Majer I, Cappelleri JC, Tarallo M. A European chart review study on early rheumatoid arthritis treatment patterns, clinical outcomes, and healthcare utilization. Rheumatol Int. 2015;35(11):1837–49.
Putrik P, Ramiro S, Kvien TK, Sokka T, Pavlova M, Uhlig T, et al. Inequities in access to biologic and synthetic DMARDs across 46 European countries. Ann Rheum Dis. 2014;73(1):198–206.
Putrik P, Ramiro S, Kvien TK, Sokka T, Uhlig T, Boonen A, et al. Variations in criteria regulating treatment with reimbursed biologic DMARDs across European countries. Are differences related to country’s wealth? Ann Rheum Dis. 2014;73(11):2010–21.
Finckh A, Neto D, Iannone F, Loza E, Lie E, van Riel P, et al. The impact of patient heterogeneity and socio-economic factors on abatacept retention in rheumatoid arthritis across nine European countries. RMD Open. 2015;1:e40.
Rintelen B, Zwerina J, Herold M, Singer F, Hitzelhammer J, Halder W, et al. Validity of data collected in BIOREG, the Austrian register for biological treatment in rheumatology: current practice of bDMARD therapy in rheumatoid arthritis in Austria. BMC Musculoskelet Disord. 2016. https://doi.org/10.1186/s12891-016-1207-4
Leon L, Rodriguez-Rodriguez L, Rosales Z, Gomez A, Lamas JR, Pato E, et al. Long-term drug survival of biological agents in patients with rheumatoid arthritis in clinical practice. Scand J Rheumatol. 2016;45(6):456–60.
Acknowledgements
The authors would like to thank all patients and the physicians in private practice in Linz, Klagenfurt, and Villach, and at the following sites: EKH Wien, Wien; LKH Vöcklabruck, Vöcklabruck; St-Johanns-Spital, Salzburg; KH der Barmherzigen Brüder Eggenberg, Graz; LKH Bludenz, Bludenz; Universitätsklinik für Innere Medizin, Innsbruck; and Rheumapraxis and Gammaswingzentrum, Hall in Tirol, who participated in the ACTION study.
Funding
The study was sponsored by Bristol-Myers Squibb (Study number: NCT02109666). Syneos Health was the contract research organization for this study and StatProcess performed the statistical analysis. Professional medical writing and editorial assistance was provided by Fiona Boswell, PhD, at Caudex and was funded by Bristol-Myers Squibb.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Galeazzi declares that he has no competing interests. P. Peichl: consulting fees: Astropharma, Bristol-Myers Squibb, Eli Lilly, Merck Sharp & Dohme, Novartis, Pfizer, Roche, Sandoz; research grants: Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis. R. Alten: research grants: Bristol-Myers Squibb. H.-M. Lorenz: consulting fees: AbbVie, Actelion, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Janssen-Cilag, Medac, Merck Sharp & Dohme, Novartis, Pfizer, Roche-Chugai, Sobi, UCB. H. Nüßlein: consulting fees: AbbVie, Bristol-Myers Squibb, Celgene, Janssen, Lilly, Merck Sharp & Dohme, Novartis, Pfizer, Roche, UCB. F. Navarro: consulting fees: AbbVie, Bristol-Myers Squibb, Eli Lilly, Janssen, Merck Sharp & Dohme, Pfizer, Roche, UCB; research grants: AbbVie, Bristol-Myers Squibb, Merck Sharp & Dohme, Pfizer, Roche; speakers’ bureau: AbbVie, Bristol-Myers Squibb, Merck Sharp & Dohme, Pfizer, Roche, UCB. Y. Elbez: consulting fees: Bristol-Myers Squibb. M. Chartier, R. Hackl: employment: Bristol-Myers Squibb. C. Rauch, S.E. Connolly: employment and shareholding: Bristol-Myers Squibb.
Ethical standards
ACTION was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice guidelines, and the Good Epidemiological Practice guidelines. The study protocol and patient enrollment materials were approved by local ethics committees and review boards. All enrolled patients provided written informed consent in accordance with local laws.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Peichl, P., Alten, R., Galeazzi, M. et al. Abatacept retention and clinical outcomes in Austrian patients with rheumatoid arthritis: real-world data from the 2-year ACTION study. Wien Med Wochenschr 170, 132–140 (2020). https://doi.org/10.1007/s10354-019-00710-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10354-019-00710-8