Summary
Introduction
Recently, first reports on benefits from Enhanced Recovery After Surgery (ERAS) pathway in patients undergoing gastric surgery have appeared. It seems that maximal reduction of unfavorable surgery-induced trauma in patients with gastric malignancy via ERAS protocol combined with minimally invasive techniques can improve outcomes.
Objective
The aim of this study was to determine the influence of laparoscopic surgery and ERAS protocol in oncological gastric surgery on early outcomes.
Materials and methods
Prospective analysis involved 28 patients (18 female and 10 male) with gastric malignancy who underwent laparoscopic gastric resection between 2009 and 2013. Gastric tumors (gastrointestinal stromal tumors or adenocarcinoma) were the indication for the surgery. A total of 17 patients underwent laparoscopic local excision, and 11 patients with adenocarcinoma or multiple neuroendocrine tumors underwent laparoscopic D2 total gastrectomy. Perioperative care was based on ERAS principles. Length of hospital stay, postoperative course, perioperative complications, and readmission rates were analyzed.
Results
There was one conversion in the gastrectomy group. All patients were mobilized on the day of surgery. Oral fluids were introduced on day 0 and were well tolerated. Full hospital diet was started on day 2 in all patients, but was well tolerated in only 18 of them. One postoperative complication requiring reoperation was noted. The length of stay after gastrectomy and gastric wedge resection was 4.6 (2–6) and 3.3 (2–6) days, respectively. No readmissions were noted in the entire group.
Conclusions
The implementation of ERAS protocol to clinical practice in combination with laparoscopy in patients with gastric tumors can result in improved postoperative care quality, shortening of hospital stay, and quicker return to normal activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Enhanced Recovery After Surgery (ERAS) concept of multimodal perioperative care was proposed by Henrik Kehlet almost 2 decades ago and continues to gain support among surgeons from all over the world [1]. Its use effectively decreases surgery-induced trauma and postoperative intestinal insufficiency. In addition to appropriate analgesia, early postoperative oral feeding and mobilization allow for a shortened hospital stay while decreasing the number of complications [2]. The ERAS protocol is well documented in colorectal surgery by several randomized controlled trials, which confirmed its effectiveness [3]. Recently, the first reports on benefits from this kind of perioperative care in patients undergoing gastric surgery have appeared [4]. In the past years, there has been an increase in the number of laparoscopic surgeries performed due to gastrointestinal (GI) neoplasms. In comparison with open surgery, laparoscopy is associated with less postoperative pain, quicker recovery, and better quality of life early after the procedure [4, 5]. As in other operations, it is associated with better postoperative outcomes when compared with the classical approach [6]. This is especially important to patients with gastric neoplasms, particularly cancer, who are usually in poor general condition—commonly undernourished and burdened by neoadjuvant chemotherapy and comorbidities. It seems that a maximal reduction of unfavorable surgery-induced trauma in this particular group of patients via the enhanced recovery program combined with minimally invasive techniques can result in improved outcomes.
Aim
The aim of this study was to analyze patient outcomes after minimally invasive surgery for gastric cancer and the application of perioperative care according to the ERAS protocol.
Materials and methods
The analysis covered all patients who underwent laparoscopic gastric resection due to malignancy between August 2009 and November 2013. Inclusion criteria were surgery due to gastric malignancy, performed with the intention to cure; use of the laparoscopic approach; and the patient’s consent to perioperative care according to ERAS principles. The group included 28 patients (18 female and 10 male). Mean age was 64 years (range: 39–86 years), and mean body mass index was 27.4 kg/m2 (range: 16.1–39.1 kg/m2). The characteristics of the studied group are represented in Table 1.
Indications for surgery were malignant gastric tumors: GI stromal tumors (GIST) in 17 patients, adenocarcinoma in 10, and multiple neuroendocrine tumors in 1. In total, 18 gastric wedge resections and 10 gastrectomies with D2 lymphadenectomy were performed (Table 2).
Preoperative staging (computed tomography, endoscopic ultrasound) excluded distant metastases. In all patients, histological verification of the tumor was achieved preoperatively. In patients with GIST, the average tumor size was 3.5 cm (1.5–6 cm). Five of nine patients with gastric adenocarcinoma received preoperative chemotherapy (ECF protocol—three cycles of epirubicin, cisplatin, and 5-fluorouracil). Patients were eligible for neoadjuvant treatment if their World Health Organization performance status was 0 or 1 and the histologically proven adenocarcinoma of the stomach was considered to be stage II or higher, with no evidence of distant metastases, or if they had locally advanced disease. Preoperative staging was based on computed tomography. Of the four patients who had not undergone preoperative chemotherapy, two were not submitted to neoadjuvant treatment due to the early stage of the disease, and in the case of two others, chemotherapy was contraindicated due to their poor general health.
Operative technique
Gastric wedge resection
After the creation of pneumoperitoneum and introduction of four trocars, intraoperative gastroscopy was performed to precisely locate the tumor. In case of smaller tumors, wedge resection was performed using an Echelon stapler (green color). A running suture would be added for stapler line reinforcement. Larger lesions were resected with a harmonic scalpel, and the defect in the stomach was closed with two layers of running suture. None of the patients had intraoperative tumor perforation. Specimens were placed in a plastic bag and removed through minilaparotomy in the epigastrium. In four patients, small tumor size allowed for extraction of the specimen through the mouth with the assistance of gastroscopy (natural orifice specimen extraction). At the end of the surgery, the anastomosis patency test was performed via insufflation by gastroscope. Mean operative time was 68 min (40–150 min).
Gastrectomy
After the creation of a pneumoperitoneum, five trocars were introduced. The stomach was dissected according to all oncological principles to obtain appropriate lymphadenectomy. Side-to-side esophagoenterostomy with a Roux limb was then performed using a linear stapling device, and the anterior wall was closed by two absorbable running stitches. Subsequently, entero-enteral side-to-side anastomosis was performed with a similar technique. The specimen was placed in a plastic bag and then removed through minilaparatomy, additionally protected with a wound protector. A blue dye leakage test of the esophagoenteric anastomosis was performed at the end of surgery. In some patients, after hemostasis, a drain was left in the area of the duodenal stump.
In one patient with cardiac infiltration involving 6 cm of distal esophagus, it was necessary to dissect the larger part of the esophagus to allow for an adequate proximal resection margin. In another patient with multiple neuroendocrine tumors also involving the duodenal bulb, the margins of the distal resection were widened.
Perioperative care
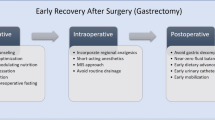
Patients were admitted to the hospital on the day preceding surgery. On admission, they were provided with detailed information on the planned treatment and perioperative care. Two hours before the surgery, all patients received 400 ml of a carbohydrate-rich drink. Operations were performed with the laparoscopic technique. In 20 patients, minilaparotomy and trocar placement sites were postoperatively injected with 0.25 % bupivacaine solution (incisions 10 mm and greater in size were injected with 10 ml of bupivacaine, while smaller incisions with 5 ml of bupivacaine solution). In the remaining eight patients, transversus abdominis plane block (TAP block) under ultrasound guidance was used at the end of the surgery (20 ml of 0.25 % bupivacaine solution on each side). Intraoperatively, the fluid therapy was restricted, and natrium-rich crystaloids were partially replaced by colloids. All patients received prophylaxis for postoperative nausea and vomiting through the administration of 8 mg of intravenous dexamethasone and 8 mg of ondansetron prior to the end of surgery, and supplemental oxygen was administered postoperatively. No nasogastric tubes were left after the surgery. Drains were used only in selected cases (not routinely). As patients returned to the ward, oral fluid intake was introduced. Intravenous fluid administration was ceased if the patient could tolerate oral fluids. A single shot of antibiotic was administered for a second time on the first postoperative day (cefazoline 2 g). All patients were mobilized on the day of surgery. Analgesia was based on paracetamol and nonsteroidal anti-inflammatory drugs (NSAIDs). In the early postoperative hours, morphine could be administered in minimal doses on patient’s demand (patient-controlled anesthesia). Upper GI contrast X-ray series with water-soluble contrast were performed for esophagojejunostomy leakage test on the first postoperative day. None of the patients had stenosis or anastomotic leakage. Afterward, the diet was expanded gradually to a full hospital diet with additional supplementation of protein-rich drinks.
Results
Conversion was necessary in one case because the infiltration of the tumor could not be assessed. In that particular case, total gastrectomy with splenectomy and resection of the transverse colon were performed. In another patient, an accidental splenic artery injury occurred during dissection of neighboring tissues by a Ligasure vessel sealer. The vessel was successfully sutured, but due to the suturing delay and signs of thrombosis in the vessel, splenectomy had to be performed. There were no intraoperative complications in the remaining patients. Mean time of gastrectomy was 233 min (160–420 min), and wedge resection, 75 min (35–140 min). R0 resection was confirmed in all patients. In the case of D2 gastrectomy, the mean number of harvested lymph nodes was 29.2 (22–36), and to our knowledge, it is comparable with results from open surgery in our center.
Postoperative complications occurred in one patient in whom reoperation was required after gastric wedge resection, due to problems with the passage of gastric contents resulting from stenosis at the suture line. Oral fluids were introduced on day 0 and were well tolerated in 26 (92.8 %) patients. Intravenous fluids were stopped within the first 24 h in 23 (82.1 %) patients. A full hospital diet was started on day 2 in all patients; however, it was well tolerated in only 18 (64.0 %) of them—in 5 from the gastrectomy group and 13 from the local excision group. All patients were mobilized (including the patient after conversion) within the first 24 h after surgery. Only five patients required postoperative doses of morphine during the first day after surgery (total dose not exceeding 20 mg). Urinary catheters were removed on the day of surgery in the afternoon in all but the one converted patient. There was no urinary retention in the study group. Drains in the peritoneal cavity were left in five patients after gastrectomy—we removed them on the first postoperative day in three cases, while for the remaining two patients, the drains were left in because the intraoperative blue dye leakage test indicated a need for anastomosis reinforcement with an additional layer of sutures; these drains were removed on the second postoperative day. The length of the hospital stay in patients after gastrectomy and gastric wedge resection was 4.6 (2–6) days and 3.3 (2–6) days, respectively. No early postoperative complications or readmissions were noted. Histological results confirmed preoperative diagnoses in all patients.
Discussion
The most recent data confirm that the oncological quality of laparoscopic resection of GISTs is comparable with open surgery and provides better postoperative outcomes and a lower risk of complications. Thus, many experts agree that minimally invasive techniques should be the first-choice treatment option in patients with GIST [7–9]. We also know that laparoscopy is a safe alternative for patients with gastric adenocarcinoma undergoing gastrectomy [10]. A comparison of laparoscopic surgery and open operations shows that the number of harvested lymph nodes is similar regardless of the chosen technique [11–13].
In our study, perioperative care was based on the guidelines of the ERAS protocol. It is well known that perioperative care of this kind benefits patients after colonic resections [14]. ERAS is widely used in bariatric surgery and allows for shortening hospital stays after surgical procedures, even down to 1 day [15]. Patients undergoing gastric surgery due to malignancy represent a specific group. They are commonly burdened by neoadjuvant systemic chemotherapy, have numerous comorbidities, and are frequently undernourished. All these factors can increase the risk of early postoperative complications that can postpone adjuvant therapy and worsen overall treatment outcomes. We can reasonably expect a decrease in unfavorable surgery-induced trauma through implementation of the ERAS protocol. Currently, only a few publications report improved postoperative outcomes in laparoscopic gastric surgery as a result of enhanced recovery programs [16]. These publications unambiguously emphasize the shorter hospital stays that result from using the protocol, though only a few of them report an accompanying lower rate of complications [16].
Although fasting even up to 12 h prior to surgery has been a standard practice intended to prevent pulmonary aspiration in elective surgery, recent studies have found no scientific support for its effectiveness. A review of available studies and clinical observations provided robust evidence that reducing preoperative fasting for clear fluids to 2 h and solid foods to 6 h does not lead to an increase in complication rates. Additionally, administration of carbohydrate-rich fluids 2–3 h prior to surgery reduces the fasting time, which decreases catabolic reaction and reduces insulin resistance in the organism, has an overall positive influence on the nitrogen balance, and also allows for earlier postoperative return of GI function [17, 18]. It is also proven to be safe in patients with uncomplicated diabetes mellitus type 2 [19].
In the postoperative period, fluid intake can be started on the day of the surgery so that on the second postoperative day, patients receive a full semi-liquid diet. In many centers, postoperative oral intake tends to be restricted; however, there is strong evidence that early postoperative enteral feeding (on the day of surgery) reduces postoperative catabolism, hastens the return of intestinal function, and decreases the risk of complications (as well as reduces the risk of anastomotic leakage). This was most extensively studied in colorectal surgery [20, 21]. There are far fewer publications available on similar topics in the case of upper GI tract surgery. According to Lassen et al. [22], who performed a multicenter randomized control trial on upper GI surgery patients, the early introduction of a full diet on the first postoperative day was not associated with a higher complication rate and allowed for a reduction in the hospital stay. To sum up, it seems that early oral intake, starting with a small amount of mixed food or protein-rich drinks, results in faster return of GI tract function and reduces the length of the hospital stay. Additionally, early enteral feeding allows for the reduction of postoperative intravenous fluid administration, which has positive effect on the return of bowel function and lowers the complication rate [23–25]. The presented group of patients was allowed to drink up to 800 ml of fluids on the day of the surgery, which partially eliminated the need of infusions. No advantage to routine use of nasogastric tube has been observed. It did not decrease anastomotic dehiscence risk, the number of pulmonary complications, or the mortality rate. A meta-analysis by Yang et al. [26] including more than 700 patients after gastrectomy has shown that leaving of esophageoenteric tube prolongs postoperative ileus and the time of the first passage of flatus. Additionally, it significantly decreases the patient’s postoperative comfort.
Evidence also shows no benefit in the use of drains in the case of colorectal surgery, or surgery of the pancreas, appendix, or gallbladder [27, 28]. No publications on this topic were found with regard to upper GI surgery. The advantage of minimally invasive techniques can be that relaparoscopy in the case of suspected bleeding or anastomotic dehiscence is associated with lower complication risk than relaparotomy [4]. In the described group of patients, drains were left in only selected cases. Minimal postoperative drainage allowed for their early removal. Although we left them in the first few cases, we tend not to use them anymore in any uncomplicated surgery.
Analgesia plays a fundamental role in postoperative care. Epidural anesthesia is particularly important in the case of classical operations, but its use in laparoscopy is limited. Increasingly common is the use of trocar placement sites for infiltration with local anesthetic drugs or TAP block [29]. Evidence has shown that analgesia based on NSAIDs is generally sufficient in the case of laparoscopic surgeries [30, 31]. In our opinion, NSAIDs together with paracetamol and local anesthesia (especially TAP block) can provide full pain control in the first few days after surgery. When needed, analgesia can be enhanced with small doses of opioids (patient controlled analgesia).
An important element of the ERAS protocol is early mobilization [32]. Its importance is well documented in the study by Smart et al. [33], which showed that failure of early mobilization is one of the most common causes of deviation from the ERAS protocol and is associated with prolonged hospital stays. Early effective postoperative mobilization of patients is possible mainly due to adequate pain control. Similarly, restriction of intravenous fluid administration, early removal of the urinary catheter, and peritoneal drains improve postoperative patients’ comfort, leading to earlier postoperative mobilization.
Conclusions
In our opinion, the clinical implementation of the ERAS protocol in combination with laparoscopy for patients operated on due to malignant gastric tumors can result in improved postoperative care quality, shortened hospital stays, and a faster return to normal activity in the postoperative period.
Consent
Informed consent was obtained from all patients for being included in the study.
Conflict of interest
Michał Pędziwiatr, Maciej Matłok, Mikhail Kisialeuski, Marcin Migaczewski, Piotr Major, Marek Winiarski, Piotr Budzynński, Anna Zub-Pokrowiecka, and Andrzej Budzynński have no conflicts of interest to declare.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78(5):606–17.
Nygren J, Thacker J, Carli F, et al. Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS(®)) Society recommendations. World J Surg. 2013;37(2):285–305.
Zhuang CL, Ye XZ, Zhang XD, et al. Enhanced recovery after surgery programs versus traditional care for colorectal surgery: a meta-analysis of randomized controlled trials. Dis Colon Rectum. 2013;56(5):667–78.
Grantcharov TP, Kehlet H. Laparoscopic gastric surgery in an enhanced recovery programme. Br J Surg. 2010;97(10):1547–51.
Adachi Y, Suematsu T, Shiraishi N, et al. Quality of life after laparoscopy-assisted Billroth I gastrectomy. Ann Surg. 1999;229(1):49–54.
Matsuhashi N, Osada S, Yamaguchi K, et al. Oncologic outcomes of laparoscopic gastrectomy: a single-center safety and feasibility study. Surg Endosc. 2013;27(6):1973–9.
Ohtani H, Maeda K, Noda E, et al. Meta-analysis of laparoscopic and open surgery for gastric gastrointestinal stromal tumor. Anticancer Res. 2013;33(11):5031–41.
Miettinen M, Furlong M, Sarlomo-Rikala M, Burke A, Sobin LH, Lasota J. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the rectum and anus: a clinicopathologic, immunohistochemical, and molecular genetic study of 144 cases. Am J Surg Pathol. 2001;25:1121–33.
Karakousis GC, Singer S, Zheng J, et al. Laparoscopic versus open gastric resections for primary gastrointestinal stromal tumors (GISTs): a size-matched comparison. Ann Surg Oncol. 2011;18(6):1599–605.
Xiong JJ, Nunes QM, Huang W, et al. Laparoscopic vs open total gastrectomy for gastric cancer: a meta-analysis. World J Gastroenterol. 2013;19(44):8114–32. doi:10.3748/wjg.v19.i44.8114.
Du J, Zheng J, Li Y, et al. Laparoscopy-assisted total gastrectomy with extended lymph node resection for advanced gastric cancer—reports of 82 cases. Hepatogastroenterology. 2010;57(104):1589–94.
Cui M, Xing JD, Yang W, et al. D2 dissection in laparoscopic and open gastrectomy for gastric cancer. World J Gastroenterol. 2012;18(8):833–9.
Wei HB, Wei B, Qi CL, et al. Laparoscopic versus open gastrectomy with D2 lymph node dissection for gastric cancer: a meta-analysis. Surg Laparosc Endosc Percutan Tech. 2011;21(6):383–90.
Gustafsson UO, Scott MJ, Schwenk W, et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations. World J Surg. 2013;37(2):259–84.
Lemanu DP, Singh PP, Berridge K, et al. Randomized clinical trial of enhanced recovery versus standard care after laparoscopic sleeve gastrectomy. Br J Surg. 2013;100(4):482–9. doi:10.1002/bjs.9026.
Dorcaratto D, Grande L, Pera M. Enhanced recovery in gastrointestinal surgery: upper gastrointestinal surgery. Dig Surg. 2013;30(1):70–8.
Nygren J, Soop M, Thorell A, et al. Preoperative oral carbohydrate administration reduces postoperative insulin resistance. Clin Nutr. 1998;17(2):65–71.
Svanfeldt M, Thorell A, Hausel J, et al. Randomized clinical trial of the effect of preoperative oral carbohydrate treatment on postoperative whole-body protein and glucose kinetics. Br J Surg. 2007;94(11):1342–50.
Breuer JP, von Dossow V, von Heymann C, et al. Preoperative oral carbohydrate administration to ASA III-IV patients undergoing elective cardiac surgery. Anesth Analg. 2006;103(5):1099–108.
Lewis SJ, Egger M, Sylvester PA, et al. Early enteral feeding versus “nil by mouth” after gastrointestinal surgery: systematic review and meta-analysis of controlled trials. BMJ. 2001;323(7316):773–6.
Han-Geurts IJ, Hop WC, Kok NF, et al. Randomized clinical trial of the impact of early enteral feeding on postoperative ileus and recovery. Br J Surg. 2007;94(5):555–61.
Lassen K, Kjaeve J, Fetveit T, et al. Allowing normal food at will after major upper gastrointestinal surgery does not increase morbidity: a randomized multicenter trial. Ann Surg. 2008;247(5):721–9.
Varadhan KK, Lobo DN. A meta-analysis of randomised controlled trials of intravenous fluid therapy in major elective open abdominal surgery: getting the balance right. Proc Nutr Soc. 2010;69(4):488–98.
Lobo DN, Bostock KA, Neal KR, et al. Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: a randomised controlled trial. Lancet. 2002;359(9320):1812–8.
Nisanevich V, Felsenstein I, Almogy G, et al. Effect of intraoperative fluid management on outcome after intraabdominal surgery. Anesthesiology. 2005;103(1):25–32.
Yang Z, Zheng Q, Wang Z. Meta-analysis of the need for nasogastric or nasojejunal decompression after gastrectomy for gastric cancer. Br J Surg. 2008;95(7):809–16.
Petrowsky H, Demartines N, Rousson V, et al. Evidence-based value of prophylactic drainage in gastrointestinal surgery: a systematic review and meta-analyses. Ann Surg. 2004;240(6):1074–84. Discussion 1084–5.
Kawai M, Tani M, Terasawa H, et al. Early removal of prophylactic drains reduces the risk of intra-abdominal infections in patients with pancreatic head resection: prospective study for 104 consecutive patients. Ann Surg. 2006;244(1):1–7.
Sinha A, Jayaraman L, Punhani D. Efficacy of ultrasound-guided transversus abdominis plane block after laparoscopic bariatric surgery: a double blind, randomized, controlled study. Obes Surg. 2013;23:548–53.
Neudecker J, Schwenk W, Junghans T, et al. Randomized controlled trial to examine the influence of thoracic epidural analgesia on postoperative ileus after laparoscopic sigmoid resection. Br J Surg. 1999;86(10):1292–5.
Baca B, Gonenc M, Hamzaodlu I, et al. Randomized clinical trial comparing epidural anaesthesia and patient-controlled analgesia after laparoscopic segmental colectomy (Br J Surg 2003; 90: 1195–1199). Br J Surg. 2004;91(1):125. Author reply 125.
Vlug MS, Wind J, Hollmann MW, et al. Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: a randomized clinical trial (LAFA-study). Ann Surg. 2011;254(6):868–75.
Smart NJ, White P, Allison AS, et al. Deviation and failure of enhanced recovery after surgery following laparoscopic colorectal surgery: early prediction model. Colorectal Dis. 2012;14(10):e727–34.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pędziwiatr, M., Matłok, M., Kisialeuski, M. et al. Short hospital stays after laparoscopic gastric surgery under an Enhanced Recovery After Surgery (ERAS) pathway: experience at a single center. Eur Surg 46, 128–132 (2014). https://doi.org/10.1007/s10353-014-0264-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10353-014-0264-x