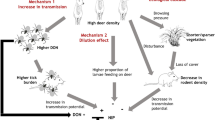
Abstract
Control of livestock diseases can become complicated when wild animals are involved. The Eurasian badger (Meles meles) is considered the principle wildlife host of Mycobacterium bovis (which causes bovine tuberculosis, bTB) in Great Britain and Ireland, but wild deer have also been implicated. Whether wild deer are likely to perpetuate bTB in cattle depends on the exposure risks they pose, the mode of pathogen transmission, the distances over which the disease can be transported and whether they can maintain infection within their own populations independently of other sources. We evaluated the likely host status of each of four species of wild British deer (red, roe, fallow and Reeves' muntjac) and the badger across a range of densities typically observed in Britain by manipulating the reproductive number equation proposed by Anderson and May (1991). We estimate that roe deer almost certainly act as spillover hosts at densities lower than 30 km−2, red deer below 16 km−2, muntjac below 6 km−2, fallow below 4 km−2 and the badger below 2 km−2. We also estimate that muntjac will almost certainly act as maintenance hosts at densities above 56 km−2, fallow above 47 km−2 and badgers above 24 km−2. For densities between these values, we cannot be certain of the host status of these species, and for red and roe deer we cannot be certain of host status under most natural conditions typically experienced in parts of Britain experiencing high incidence of bTB in cattle. However, enhanced transmission rates resulting from artificially high densities such as might be experienced at supplementary feeding sites may be sufficient to promote independent maintenance of infection. We were not able to confidently assign host status to any species over a wide range of densities, but conclude that this is likely to reflect reality, where host status may be affected as much by, for example, demographic fluctuations as it is by population density. Our results imply densities below which populations of wild deer inhabiting cattle bTB hotspots ought to be maintained in order to control the possibility of them perpetuating the cycle of intra- and interspecific M. bovis transmission.


Similar content being viewed by others
References
Acevedo P, Ward AI, Real R, Smith GC (2010) Assessing biogeographical relationships in a multi-species system using favourability functions: a case study on British deer. Divers Distrib 16:515–528
Anderson RM, May RM (1991) Infectious diseases in humans. Dynamics and control. Oxford University Press, New York
Barlow ND (1991) A spatially aggregated disease/host model of bovine TB in New Zealand possum populations. J Appl Ecol 28:777–793
Barlow ND (2000) Non-linear transmission and simple models for bovine tuberculosis. J Anim Ecol 69:703–713
Caley P, Hone J (2005) Assessing the host disease status of wildlife and the implications for disease control: Mycobacterium bovis infection in feral ferrets. J Appl Ecol 42:708–719
Carter SP, Roy SS, Cowan DP, Massei G, Smith GC, Ji W, Rossi S, Woodroffe R, Wilson GJ, Delahay RJ (2009) Options for the control of disease 2: targeting hosts. In: Delahay RJ, Smith GC, Hutchings MR (eds) Management of disease in wild mammals. Springer, Tokyo, pp 121–146
Central Science Laboratory (2006) A quantitative risk assessment on the role of wild deer in the perpetuation of TB in cattle. Project report SE3036. Defra, London
Chapman NG, Claydon K, Claydon M, Forde PG, Harris S (1993) Sympatric populations of muntjac (Muntiacus reevesi) and roe deer (Capreolus capreolus)—a comparative analysis of their ranging behaviour, social organization and activity. J Zool (Lond) 229:623–640
Clutton-Brock T (1989) Mammalian mating systems. Proc R Soc Lond 236B:339–372
Cox DR, Donnelly CA, Bourne FJ, Gettinby G, McInerney JP, Morrison WI, Woodroffe R (2005) Simple model for tuberculosis in cattle and badgers. PNAS 102:17588–17593
Crawshaw TR, Griffiths IB, Clifton-Hadley RS (2008) Comparison of a standard and a detailed postmortem protocol for detecting Mycobacterium bovis in badgers. Vet Rec 163:473–477
Cross PC, Drewe J, Patrek V, Pearce G, Samuel MD, Delahay RJ (2009) Wildlife population structure and parasite transmission: implications for disease management. In: Delahay RJ, Smith GC, Hutchings MR (eds) Management of disease in wild mammals. Springer, Tokyo, pp 9–29
de Lisle GW, Mackintosh CG, Bengis RG (2001) Mycobacterium bovis in free-living and captive wildlife, including farmed deer. Rev Sci Tech Off Int Epiz 20:86–111
de Lisle GW, Bengis RG, Schmitt SM, O'Brien DJ (2002) Tuberculosis in free-ranging wildlife: detection, diagnosis and management. Rev Sci Tech Off Int Epiz 21:317–334
Delahay RJ, de Leeuw ANS, Barlow AM, Clifton-Hadley RS, Cheeseman CL (2002) The status of Mycobacterium bovis infection in British wild mammals: a review. Vet J 163:1–16
Delahay RJ, Smith GC, Ward AI, Cheeseman CL (2005) Options for the management of bovine tuberculosis transmission from badgers (Meles meles) to cattle: evidence from a long-term study. Mamm Stud 30:S73–S81
Delahay RJ, Smith GC, Barlow AM, Walker N, Harris A, Clifton-Hadley RS, Cheeseman CL (2007) Bovine tuberculosis infection in wild mammals in the south-west region of England: a survey of prevalence and a semi-quantitative assessment of the relative risks to cattle. Vet J 173:287–301
Donnelly CA, Woodroffe R, Cox DR, Bourne FJ, Cheeseman CL, Clifton-Hadley RS, Wei G, Gettinby G, Gilks P, Jenkins H, Johnston WT, Le Fevre AM, McInerney JP, Morrison WI (2005) Positive and negative effects of widespread badger culling on tuberculosis in cattle. Nature 439:843–846
Ferretti F, Sforzi A, Lovari S (2008) Intolerance amongst deer species at feeding: roe deer are uneasy banqueters. Behav Process 78:487–491
Gallagher J, Clifton-Hadley RS (2000) Tuberculosis in badgers; a review of the disease and its significance for other animals. Res Vet Sci 69:203–217
Green P (2004) Tuberculosis in wild deer on Exmoor. Deer 13:14–17
Griffin JFT, Mackintosh CG (2000) Tuberculosis in deer: perceptions, problems and progress. Vet J 160:202–219
Griffin JFT, Chin DN, Rodgers CR (2004) Diagnostic strategies and outcomes on those New Zealand deer farms with severe outbreaks of bovine tuberculosis. Tuberculosis 84:293–302
Griffin JM, William DH, Kelly GE, Clegg TA, O'Boyle I, Collins JD, More SJ (2005) The impact of badger removal on the control of tuberculosis in cattle herds in Ireland. Prev Vet Med 67:237–266
Harris S, Yalden DW (2008) Mammals of the British isles: handbook, 4th edn. The Mammal Society, Southampton
Hars J, Boschiroli ML, Duvauchelle A, Zanella G, Garin-Bastuji B (2007) Bovine tuberculosis in free-living wild ungulates in France. In: Book of abstracts, Ecology and Management of Wildlife Diseases Conference, Central Science Laboratory, York, p 15
Hayden DT, Cleaveland S, Taylor LH, Laurenson MK (2002) Identifying reservoirs of infection: a conceptual and practical challenge. Emerg Infect Dis 8:1468–1473
Hill JA (2005) Wildlife–cattle interactions in northern Michigan: implications for the transmission of bovine tuberculosis. MS thesis, Utah State University
Independent Scientific Group on Cattle TB (ISG) (2007) Bovine TB: the scientific evidence. A science base for a sustainable policy to control TB in cattle. An epidemiological investigation into bovine tuberculosis. Report to the Rt Hon David Miliband. London, Defra
Langbein J (2008) Management cull, not mass slaughter. Deer 14:22–23
Lloyd-Smith JO, Cross PC, Briggs CJ, Daugherty M, Getz WM, Latto J, Sanchez MS, Smith AB, Swei A (2005) Should we expect population thresholds for wildlife disease? TREE 20:511–519
McCallum H, Barlow N, Hone J (2001) How should pathogen transmission be modelled? TREE 16:295–300
Mysterud A (1998) Large male territories in a low-density population of roe deer Capreolus capreolus with small female home ranges. Wildl Biol 4:231–235
Nugent G (2005) The role of wild deer in the epidemiology and management of bovine tuberculosis in New Zealand. PhD thesis, Lincoln University
Palmer MV, Waters MR, Whipple DL (2004a) Shared feed as a means of deer-to-deer transmission of Mycobacterium bovis. J Wildl Dis 40:87–91
Palmer MV, Waters WR, Whipple DL (2004b) Investigation of the transmission of Mycobacterium bovis from deer to cattle through indirect contact. Amer J Vet Res 65:1483–1489
Putman RJ, Moore NP (1998) Impact of deer in lowland Britain on agriculture, forestry and conservation habitats. Mamm Rev 28:141–164
Putman RJ, Langbein J, Hewison AJM, Sharma SK (1996) Relative roles of density-dependent and density-independent factors in population dynamics of British deer. Mamm Rev 26:81–101
Rogers LM, Cheeseman CL, Mallinson PJ, Clifton-Hadley RS (1997) The demography of a high-density badger (Meles meles) population in the west of England. J Zool (Lond) 242:705–728
Rogers LM, Delahay RJ, Cheeseman CL, Smith GC, Clifton-Hadley RS (1999) The increase in badger (Meles meles) density at Woodchester Park, south-west England: implications for disease (Mycobacterium bovis) prevalence. Mammalia 63:183–192
Rudolph BA, Riley SJ, Hickling GJ, Frawley BJ, Garner MS, Winterstein SR (2006) Regulating hunter baiting for white tailed deer in Michigan: biological and social considerations. Wildl Soc Bull 34:314–321
Sleeman DP, Davenport J, More SJ, Clegg TA, Collins JD, Martin SW, Williams DH, Griffin JM, O'Boyle I (2009) How many Eurasian badgers Meles meles L. are there in the Republic of Ireland? Eur J Wildl Res 55:333–344
Smith GC (2001) Models of Mycobacterium bovis in wildlife and cattle. Tuberculosis 81:51–64
Staines BW, Welch D (1984) Habitat selection and impact of red (Cervus elaphus L.) and roe (Capreolus capreolus L.) deer in a sitka spruce plantation. Proc Roy Soc Edinb 82B:303–319
Ward AI (2005) Expanding ranges of wild and feral deer in Great Britain. Mamm Rev 35:165–173
Ward AI, Smith GC, Etherington TR, Delahay RJ (2009) Estimating the risk of cattle exposure to TB by wild deer, relative to badgers in England and Wales. J Wildl Dis 45:1104–1120
Welch D, Staines BW, Catt DC, Scott D (1990) Habitat usage by red (Cervus elaphus) and roe (Capreolus capreolus) deer in a Scottish sitka spruce plantation. J Zool (Lond) 221:253–276
Werckman C, Hardy T, Wainwright E (2000) Crystal Ball 2000 user manual. Decisioneering Inc., Denver
Wilkinson D, Smith GC, Delahay R, Rogers LM, Cheeseman CL, Clifton-Hadley RS (2000) The effects of bovine tuberculosis (Mycobacterium bovis) on mortality in a badger (Meles meles) population in England. J Zool (Lond) 250:389–395
Woodroffe R, Donnelly CA, Wei G, Cox DR, Bourne FJ, Burke T, Butlin RK, Cheeseman CL, Gettinby G, Gilks P, Hedges S, Jenkins HE, Johnston WT, McInerney JP, Morrison WI, Pope LC (2009) Social group size affects Mycobacterium bovis infection in European badgers (Meles meles). J Anim Ecol 78:818–827
Acknowledgements
This work was funded by the UK's Department for Environment, Food and Rural Affairs' Food and Farming Group. We are grateful to Richard (Dez) Delahay, the associate editor and two anonymous reviewers for the extremely helpful, insightful comments on earlier drafts of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Gortázar
Rights and permissions
About this article
Cite this article
Ward, A.I., Smith, G.C. Predicting the status of wild deer as hosts of Mycobacterium bovis infection in Britain. Eur J Wildl Res 58, 127–135 (2012). https://doi.org/10.1007/s10344-011-0553-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10344-011-0553-7