Abstract
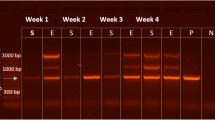
Noninvasive sampling approaches are becoming increasingly important for enabling genetic studies of wildlife populations. While a number of methods have been described to noninvasively sample hair from carnivores and medium-sized mammals, they have largely remained untested in elusive small mammals. Here we describe a novel and inexpensive noninvasive hair snare targeted at an elusive small mammal, the American pika (Ochotona princeps). We explore the quality of the sample by assessing PCR amplification success of mitochondrial and nuclear DNA fragments across four commercially available DNA isolation kits and two different quantities of hair in a factorial design. Additionally, we determined the sex of the individual samples using PCR–RFLP of ZFX/ZFY loci. We found that our snare is effective in obtaining hair that yield DNA of sufficient quality and quantity to successfully amplify a range of mitochondrial and nuclear fragment sizes. Specifically, we found the greatest success in amplifying mitochondrial DNA, nuclear microsatellites and ZFX/ZFY loci using at least 25 hairs as starting material and the DNA IQ™ system. The hair snares thus provide a cost-effective and minimally intrusive approach to sample elusive or rare small mammals. We anticipate that this approach will be useful to obtain samples for molecular studies of the ecology, evolution and conservation of small, elusive mammals.



Similar content being viewed by others
References
Banks SC, Hoyle SD, Horsup A, Sunnucks P, Taylor AC (2003) Demographic monitoring of an entire species (the northern hairy-nosed wombat, Lasiorhinus krefftii) by genetic analysis of non-invasively collected material. Anim Conserv 6:101–107
Beever EA, Brussard PE, Berger J (2003) Patterns of apparent extirpation among isolated populations of pikas (Ochotona princeps) in the Great Basin. J Mammal 84:37–54
Beja-Pereira A, Oliveira R, Alves PC, Schwartz MK, Luikart G (2009) Advancing ecological understandings through technological transformations in noninvasive genetics. Mol Ecol Resour 9:1279–1301
Bremner-Harrison S, Harrison SWR, Cypher BL, Murdoch JD, Maldonado J, Darden SK (2006) Development of a single-sampling noninvasive hair snare. Wildl Soc B 34:456–461
Broquet T, Petit E (2004) Quantifying genotyping errors in noninvasive population genetics. Mol Ecol 13:3601–3608
Connior MB, Risch TS (2009) Live trap for pocket gophers. Southwest Nat 54:100–103
DeSalle R, Amato G (2004) The expansion of conservation genetics. Nat Rev Genet 5:702–712
DeYoung RW, Honeycutt RL (2005) The molecular toolbox: genetic techniques in wildlife ecology and management. J Wildl Manage 69:1362–1384
Excoffier L, Heckel G (2006) Computer programs for population genetics data analysis: a survival guide. Nat Rev Genet 7:745–758
Fontanesi L, Tazzoli M, Pecchioli E, Hauffe HC, Robinson TJ, Russo V (2008) Sexing European rabbits (Oryctolagus cuniculus), European brown hares (Lepus europaeus) and mountain hares (Lepus timidus) with ZFX and ZFY loci. Mol Ecol Resour 8:1294–1296
Gomez A, Wright PJ, Lunt DH, Cancino JM, Carvalho GR, Hughes RN (2007) Mating trials validate the use of DNA barcoding to reveal cryptic speciation of a marine bryozoan taxon. P R Soc B 274:199–207
Hedmark E, Flagstad O, Segerstrom P, Persson J, Landa A, Ellegren H (2004) DNA-based individual and sex identification from wolverine (Gulo gulo) faeces and urine. Conserv Genet 5:405–410
Henry P, Miquelle D, Sugimoto T, McCullough DR, Caccone A, Russello MA (2009) In situ population structure and ex situ representation of the endangered Amur tiger. Mol Ecol 18:3173–3184
Kendall KC, Stetz JB, Boulanger J, Macleod AC, Paetkau D, White GC (2009) Demography and genetic structure of a recovering grizzly bear population. J Wildl Manage 73:3–17
Litvaitis JA, Tash JP, Litvaitis MK, Marchand MN, Kovach AI, Innes R (2006) A range-wide survey to determine the current distribution of New England cottontails. Wildl Soc B 34:1190–1197
Manchester KL (1995) Value of a(260)/a(280) ratios for measurement of purity of nucleic-acids. Biotechniques 19:208–210
Mitrovski P, Heinze DA, Guthridge K, Weeks AR (2005) Isolation and characterization of microsatellite loci from the Australian endemic mountain pygmy-possum, Burramys parvus Broom. Mol Ecol Notes 5:395–397
Morin PA, Chambers KE, Boesch C, Vigilant L (2001) Quantitative polymerase chain reaction analysis of DNA from noninvasive samples for accurate microsatellite genotyping of wild chimpanzees (Pan troglodytes verus). Mol Ecol 10:1835–1844
Mullins J, Statham MJ, Roche T, Turner PD, O’Reilly C (2010) Remotely plucked hair genotyping: a reliable and non-invasive method for censusing pine marten (Martes martes, L. 1758) populations. Eur J Wildl Res 56:443–453
Mullis K, Faloona F, Scharf S, Saiki R, Horn H, Erlich H (1986) Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harb Symp Quant Biol 51:263
Nachman MW (2005) The genetic basis of adaptation: lessons from concealing coloration in pocket mice. Genetica 123:125–136
Paetkau D (2003) An empirical exploration of data quality in DNA-based population inventories. Mol Ecol 12:1375–1387
Pauli JN, Hamilton MB, Crain EB, Buskirk SW (2008) A single-sampling hair trap for mesocarnivores. J Wildl Manage 72:1650–1652
Peacock MM (1997) Determining natal dispersal patterns in a population of North American pikas (Ochotona princeps) using direct mark-resight and indirect genetic methods. Behav Ecol 8:340–350
Peacock MM, Kirchoff VS, Merideth SJ (2002) Identification and characterization of nine polymorphic microsatellite loci in the North American pika, Ochotona princeps. Mol Ecol Notes 2:360–362
Piggott MP, Taylor AC (2003) Remote collection of animal DNA and its applications in conservation management and understanding the population biology of rare and cryptic species. Wildl Res 30:1–13
Piggott MP, Bellemain E, Taberlet P, Taylor AC (2004) A multiplex pre-amplification method that significantly improves microsatellite amplification and error rates for faecal DNA in limiting conditions. Conserv Genet 5:417–420
Regnaut S, Lucas FS, Fumagalli L (2006) DNA degradation in avian faecal samples and feasibility of non-invasive genetic studies of threatened capercaillie populations. Conserv Genet 7:449–453
Roon DA, Waits LP, Kendall KC (2003) A quantitative evaluation of two methods for preserving hair samples. Mol Ecol Notes 3:163–166
Roon DA, Waits LP, Kendall KC (2005) A simulation test of the effectiveness of several methods for error-checking non-invasive genetic data. Anim Conserv 8:203–215
Rudnick JA, Katzner TE, Bragin EA, DeWoody JA (2007) Species identification of birds through genetic analysis of naturally shed feathers. Mol Ecol Notes 7:757–762
Russello MA, Amato G (2001) Application of a noninvasive, PCR-Based test for sex identification in an endangered parrot, Amazona guildingii. Zoo Biol 20:41–45
Russello MA, Glaberman S, Gibbs JP, Marquez C, Powell JR, Caccone A (2005) A cryptic taxon of Galapagos tortoise in conservation peril. Biol Lett-UK 1:287–290
Sastre N, Francino O, Lampreave G, Bologov VV, Lopez-Martin JM, Sanchez A, Ramirez O (2009) Sex identification of wolf (Canis lupus) using non-invasive samples. Conserv Genet 10:555–558
Smith AT, Weston ML (1990) Ochotona princeps. Mamm Species 352:1–8
Sunnucks P (2000) Efficient genetic markers for population biology. Trends Ecol Evol 15:199–203
Taberlet P, Griffin S, Goossens B, Questiau S, Manceau V, Escaravage N, Waits LP, Bouvet J (1996) Reliable genotyping of samples with very low DNA quantities using PCR. Nucleic Acids Res 24:3189–3194
Taberlet P, Waits LP, Luikart G (1999) Noninvasive genetic sampling: look before you leap. Trends Ecol Evol 14:323–327
Toth M (2008) A new noninvasive method for detecting mammals from birds’ nests. J Wildl Manage 72:1237–1240
Waits LP, Paetkau D (2005) Noninvasive genetic sampling tools for wildlife biologists: a review of applications and recommendations for accurate data collection. J Wildl Manage 69:1419–1433
Willard JM, Lee DA, Holland MM (1998) Recovery of DNA for PCR amplification from blood and forensic samples using chelating resin. In: Lincoln PJ, Thomson J (eds) Forensic DNA profiling protocols. Humana, Totowa, pp 9–18
Woods JG, Paetkau D, Lewis D, McLellan BN, Proctor M, Strobeck C (1999) Genetic tagging of free-ranging black and brown bears. Wildl Soc B 27:616–627
Yu N, Zheng CL, Zhang YP, Li WH (2000) Molecular systematics of pikas (genus Ochotona) inferred from mitochondrial DNA sequences. Mol Phylogenet Evol 16:85–95
Acknowledgements
We would like to thank L. Evans, B. Granger, D. Rissling, Z. Sim, A. Goodwin, K. Hayhurst, D. Kuch and S. and A. Henry for assistance in the field. K. Galbreath kindly provided pika liver samples from the Bella Coola valley. Thanks to A. Gonçalves da Silva and K. Larsen for interesting discussions that contributed to the design of this noninvasive genetic sampling method. C. Miño, A. Arnall, A. Gonçalves da Silva, C. Ray, M. Peacock and two anonymous reviewers are thanked for valuable comments on the manuscript. This work was funded by the Natural Sciences and Engineering Research Council of Canada Discovery and UBC Okanagan Individual Research grants to MAR. A Swiss National Science Foundation Doctoral Fellowship PBSKP3_128523 supported PH. This study was undertaken following the animal care protocol from the University of British Columbia (certificate number A07-0126).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Gortázar
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Table S1
Information regarding mitochondrial cytochrome b fragments amplified, including fragment names, primer names and sequences as well as overall length of fragment amplified. (DOC 32 kb)
Rights and permissions
About this article
Cite this article
Henry, P., Russello, M.A. Obtaining high-quality DNA from elusive small mammals using low-tech hair snares. Eur J Wildl Res 57, 429–435 (2011). https://doi.org/10.1007/s10344-010-0449-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10344-010-0449-y