Abstract
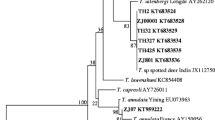
Piroplasms from Theileria genus were detected in blood and spleen of red deer Cervus elaphus culled during the months of September 2004–January 2005 in northwestern Poland. The polymerase chain reaction revealed the presence of Theileria deoxyribonucleic acid in 88% (36 of 41) of the animals examined. Molecular characterization of the parasites based on large piece of 18S ribosomal ribonucleic acid gene containing hypervariable region V4 showed 99.9% similarity to two Theileria spp. sequences: Theileria sp. 3185/02 and Theileria capreoli BAB1158. Phylogenetic analysis confirmed that the three isolates cluster together with high bootstrap support. It is supposed that those pathogens can be classified as one group characteristic for the Eurasian continent, contrary to protozoon of Theileria from the T. cervi group, which are often found on the North American continent and can also infect the representatives of Cervidae. In conclusion, this study suggested that free-living C. elaphus in northwestern Poland are a competent reservoir of Theileria sp. ZS T04 C.e. parasites, although the vector of the piroplasms is still unknown.

Similar content being viewed by others
References
Baek BK, Soo KB, Kim JH, Hur J, Lee BO, Jung JM, Onuma M, Oluoch AO, Kim CH, Kakoma I (2003) Verification by polymerase chain reaction of vertical transmission of Theileria sergenti in cows. Can J Vet Res 67:278–228
Cerny V (1958) Theileriafunde beim europäischen Hirsch im Gebiet von Topol Cianky (Slovakei). Biologia 13:509–513
Chae JS, Waghela SD, Craig T, Kocan AA, Wagner GG, Holman PJ (1999) Two Theileria cervi ssu gene sequence types found in isolates from white-tailed deer and elk in North America. J Wildl Dis 35:458–465
Danielova V, Daniel M, Rudenko N, Golovchenko M (2006) Prevalence of Borrelia burgdorferi sensu lato genospecies in host-seeking Ixodes ricinus ticks in selected South Bohemian locations (Czech Republic). Cent Eur J Public Health 12:151–156
Dehio C, Lanz C, Pohl R, Behrens P, Bermond D, Piemont Y, Pelz K, Sander A (2001) Bartonella schoenbuchii sp. nov., isolated from the blood of wild roe deer. Int J Syst Evol Microbiol 51:1557–1565
De La Fuente J, Vicente J, Hofle U, Ruiz Fons F, Fernandez De Mera IG, Van Den Bussche RA, Kocan KM, Gortazar C (2004) Anaplasma infection in free-ranging Iberian red deer in the region of Castilla-La Mancha, Spain. Vet Microbiol 100:163–173
De La Fuente J, Naranjo V, Ruiz Fons F, Hofle U, Fernandez De Mera IG, Villanua D, Almazan C, Torina A, Caracappa S, Kocan KM, Gortazar C (2005) Potential vertebrate reservoir hosts and invertebrate vectors of Anaplasma marginale and A. phagocytophilum in central Spain. Vector Borne Zoonot Dis 5:390–401
Duh D, Petrovec M, Avsic Zupanc T (2001) Diversity of Babesia infecting European sheep ticks (Ixodes ricinus). J Clin Microbiol 39:3395–3397
Duh D, Petrovec M, Bidovec A, Avsic-Zupanc T (2005) Cervids as Babesiae hosts, Slovenia. Emerg Infect Dis 11:1121–1123
Esernio-Jenssen D, Scimeca PG, Benach JL, Tenenbaum MJ (1987) Transplacental/perinatal babesiosis. J Pediatr 110:570–572
Gallatin LL, Irizarry-Rovira AR, Renninger ML, Holman PJ, Wagner GG, Sojka JE, Christian JA (2003) Babesia odocoilei infection in elk. J Am Vet Med Assoc 223:1027–1032
Gitau GK, Perry BD, McDermott JJ (1999) The incidence, calf morbidity and mortality due to Theileria parva infections in smallholder dairy farms in Murang’a District, Kenya. Prev Vet Med 39:65–79
Goethert H, Telford S (2003) Enzootic transmission of Babesia divergens among cottontail rabbits on Nantucket Island, Massachusetts. Am J Trop Med Hyg 69:455–460
Hinaidy HK (1987) Blood parasites of wild ruminants in Austria. Zentralbl Veterinarmed B 34:81–97
Höfle U, Vicente J, Nagore D, Hurtado A, Pena A, de la Fuente J, Gortazar C (2004) The risks of translocating wildlife. Pathogenic infection with Theileria sp. and Elaeophora elaphi in an imported red deer. Vet Parasitol 126:387–395
Kjemtrup AM, Thomford J, Robinson T, Conrad PA (2000) Phylogenetic relationship of human and wildlife piroplasm isolates in the western United States inferred from the 18S nuclear small subunit RNA gene. Parasitology 120:487–493
Kocan AA, Kocan KM (1991) Tick-transmitted protozoan diseases of wildlife in North America. Bull Soc Vector Ecol 16:94–108
Laird JS, Kocan AA, Kocan KM, Presley SM, Hair JA (1988) Susceptibility of Amblyomma americanum to natural and experimental infections with Theileria cervi. J Wildl Dis 24:679–683
Phipps LP, Otter A (2004) Transplacental transmission of Theileria equi in two foals born and reared in the United Kingdom. Vet Rec 154:406–408
Piccolin G, Benedetti G, Doglioni C, Lorenzato C, Mancuso S, Papa N, Ramon MC, Zasio C, Bertiato G (2006) A study of the presence of B. burgdorferi, Anaplasma (previously Ehrlichia) phagocytophilum, Ricketsia, and Babesia in Ixodes ricinus collected within the territory of Belluno, Italy. Vector Borne Zoonot Dis 6:24–31
Pieniazek N, Sawczuk M, Skotarczak B (2006) Molecular identification of Babesia parasites isolated from Ixodes ricinus ticks collected in northwestern Poland. J Parasitol 92:32–35
Polin H, Hufnagl P, Haunschmid R, Gruber F, Ladurner G (2004) Molecular evidence of Anaplasma phagocytophilum in Ixodes ricinus ticks and wild animals in Austria. J Clin Microbiol 42:2285–2286
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sawczuk M, Maciejewska A, Adamska M, Skotarczak B (2005) Roe deer (Capreolus capreolus) and red deer (Cereus elaphus) as a reservoir of protozoans from Babesia and Theileria genus in nort-western Poland. Wiad Parazytol 51:243–247
Skotarczak B, Cichocka A (2001) Isolation and amplification by polymerase chain reaction DNA of Babesia microti and Babesia divergens in ticks in Poland. Ann Agric Environ Med 8:187–189
Skotarczak B, Rymaszewska A, Wodecka B, Sawczuk M (2003) Molecular evidence of coinfection of Borrelia burgdorfei sensu lato, human granulocytic ehrlichiosis agent, and Babesia microti in ticks from northwestern Poland. J Parasitol 89:194–196
Tait A, Hall FR (1990) Theileria annulata: control measures, diagnosis and the potential use of subunit vaccines. Rev Sci Tech 9:387–403
Takahashi K, Kubota S, Kawai S, Hagiwara K, Kurosawa T, Tajima M, Sonoda M, Maede Y (1992) Babesia and Theileria protozoa detected from wild sika-deer (Cervus nippon yesoensis) in Hokkaido. J Protozool Res 2:158–164
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Wodecka B, Skotarczak B (2005) First isolation of Borrelia lusitaniae DNA from Ixodes ricinus ticks in Poland. Scand J Infect Dis 37:27–34
Yabsley MJ, Davidson WR, Stallknecht DE, Ravela AS, Swift PK, Devos JC Jr, Dubay SA (2005a) Evidence of tick-borne organisms in mule deer (Odocoileus hemionus) from the western United States. Vector Borne Zoonot Dis 5:351–362
Yabsley MJ, Quick TC, Littre SE (2005b) Theileriosis in a white-tailed deer (Odocoileus virginianus) fawn. J Wildl Dis 41:806–809
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W. Lutz
Rights and permissions
About this article
Cite this article
Sawczuk, M., Maciejewska, A. & Skotarczak, B. Identification and molecular characterization of Theileria sp. infecting red deer (Cervus elaphus) in northwestern Poland. Eur J Wildl Res 54, 225–230 (2008). https://doi.org/10.1007/s10344-007-0133-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10344-007-0133-z