Abstract
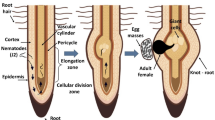
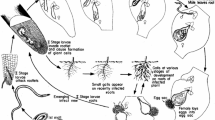
Root-knot nematodes pose a significant threat to agricultural production all over the world. They are ubiquitous, polyphagous, sedentary endoparasites and belong to genus, Meloidogyne. These biotrophic pathogens establish and maintain an intimate interaction with their host plants. Effectors are crucial for the entry of RKNs to the root of plants, subsequently leading to the formation of the specialized hypertrophied and multinucleate feeding sites for nematode growth and development. During the establishment and maintenance of nematode feeding sites, effectors are considered to play a critical role in the modulation of developmental and defence signaling pathways in host cells. Chemical nematicides are widely used in RKNs management strategies, despite their threat to environmental and human health. Recently, restrictions on using some chemicals have been enforced. So, there is a need of potential environmentally benign strategies to the management of RKNs. We will highlight most the effective strategies viz., biological control by bacteria and fungi and organic amendment by botanicals and oil cakes. Also taken into account are traditional strategies like cultural method, soil solarization and resistant cultivars. One of the best alternatives to chemical control of RKNs is biological control, which involves the use of predators and parasites to reduce the RKNs at various life stages (eggs, juveniles and adults), as well as the secretion of poisonous diffusible inhibitory metabolites which hamper the reproduction ability of RKNs. Here, we provide a brief review of the biology of the genus, Meloidogyne, plant-nematode interactions and various environmentally benign strategies.







Similar content being viewed by others
References
Abad P, Williamson VM (2010) Plant nematode interaction: a sophisticated dialogue. In: Advances in botanical research, vol 53. Academic Press, pp 147–192
Abad P, Gouzy J, Aury JM, Castagnone-Sereno P, Danchin EG, Deleury E, Wincker P (2008) Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat Biotechnol 26:909–915
Abbasi M, Ahmed N, Zaki M, Shuakat S, Khan D (2014) Potential of Bacillus species against Meloidogyne javanica parasitizing eggplant (Solanum melongena L.) and induced biochemical changes. Plant Soil 375:159–173
Abd-Elgawad MMM (2016) Biological control agents of plant-parasitic nematodes. Egypt J Biol Pest Control 26(2):423–429
AbdelRazek GM, Yaseen R (2020) Effect of some rhizosphere bacteria on root-knot nematodes. Egypt J Bio Pest Control 30:1–11
Ahmad G, Nishat Y, Ansari M, Khan A, Haris M, Khan AA (2021) Eco-friendly approaches for the alleviation of root-knot nematodes. In: Plant growth-promoting microbes for sustainable biotic and abiotic stress management. Springer, Cham, pp 557–575
Ahmad G, Khan A, Ansari S, Khan AA, Elhakem A, Sami R, Mohhamad HI (2022) Management of root-knot nematode infection by using fly ash and Trichoderma harzianum in Capsicum annum plants by modulating growth, yield, photosynthetic pigments, biochemical substances, and secondary metabolite profiles. Not Bot Horti Agrobot Cluj-Napoca 50:12591–12591
Aish RKNMJ, Ammar SA, Masoud SI (2015) A potential biocontrol and PGPR activities of bacteria Providencia vermicola against. J Nematol 47:218–281
Ali N, Tavoillot J, Mateille T, Chapuis E, Besnard G, El Bakkali A, Palomares-Rius JE (2015) A new root-knot nematode Meloidogyne spartelensis n. sp.(Nematoda: Meloidogynidae) in Northern Morocco. Eur J Plant Pathol 143:25–42
Almaghrabi OA, Massoud SI, Abdelmoneim TS (2013) Influence of inoculation with plant growth promoting rhizobacteria (PGPR) on tomato plant growth and nematode reproduction under greenhouse conditions. Saudi J Biol Sci 20(1):57–61
Alvarez-Ortega S, Brito JA, Subbotin S (2019) Multigene phylogeny of root-knot nematodes and molecular characterization of Meloidogyne nataliei Golden, Rose & Bird, 1981 (Nematoda: Tylenchida). Sci Rep 9:1–11
Andres MF, Gonzalez-Coloma A, Sanz J, Burillo J, Sainz P (2012) Nematicidal activity of essential oils: A review. Phytochem Rev 11:371–390
Ansari J, Sharma CP, Sagar A (2016) Interactive effect of bio-fertilizers viz., rhizobium, PSB and VAM on nitrogen, phosphorus and protein content in lentil (Lens culinaris L.) crop. Res Environ Life Sci 9:66–68
Archana S, Goswami BK (2017) Performance of leaves and their respective oil seed cakes on plant growth parameters and soil population of root knot nematode on okra. Plant Arch 17:1055–1057
Asif M, Khan A, Tariq M, Siddiqui MA (2016) Sustainable management of root knot nematode Meloidogyne incognita through organic amendment on Solanum lycopersicum L. Asian J Bio 1:1–8
Asif M, Tariq M, Khan A, Siddiqui MA (2017) Biocidal and antinemic properties of aqueous extracts of Ageratum and Coccinia against root-knot nematode, Meloidogyne incognita in vitro. J Agric Sci 12:108–123
Bacha N, Ayub N, Ahmad Y, Abbas M, Rafi A (2007) Soil solarization: a safe, affective and practicable technique for the control of soil born fungi e nematodes. Pak J Biol Sci 10:57–64
Baheti BL, Bhati SS, Singh H (2019) Efficacy of different oil-cakes as soil amendment for the management of root-knot nematode, Meloidogyne incognita infecting okra (Abelmoschus esculentus L.). Int J Curr Microbiol App Sci 8:799–808
Bailey DM (1941) The seedling test method for root-knot-nematode resistance. In: Proceedings of the American Society for Horticultural Science, vol 38, pp 573–575
Bakr RA, Ketta HA (2018) Nematicidal potential of some botanical products against Meloidogyne incognita infecting eggplant. Ind J Nematol 48:203–211
Bardgett RD, Van Der Putten WH (2014) Belowground biodiversity and ecosystem functioning. Nature 515:505–511
Barron GL (1977) The nematode-destroying fungi. Canadian Biological Publications
Barros AF, Campos VP, Souza LN, Costa SS, Terra WC, Lessa JH (2018) Morphological, enzymatic and molecular characterization of root-knot nematodes parasitizing vegetable crops. Hortic Bras 36:473–479
Bellafiore S, Shen Z, Rosso MN, Abad P, Shih P, Briggs SP (2008) Direct identification of the Meloidogyne incognita secretome reveals proteins with host cell reprogramming potential. PLoS Pathog 4:1000192
Bello A, Arias M, Lopez-Perez JA, Garcia-Alvarez A, Fresno J, Escuer M, Martinez C (2004) Biofumigation, fallow, and nematode management in vineyard replant. Nematropica 34(1):53–64
Bengtsson T (2015) Biological control of root-knot nematodes (Meloidogyne spp.) by the fungus Pochonia chlamydosporia
Blanc-Mathieu R, Perfus-Barbeoch L, Aury JM, Da Rocha M, Gouzy J, Sallet E, Danchin EG (2017) Hybridization and polyploidy enable genomic plasticity without sex in the most devastating plant-parasitic nematodes. PLoS Genet 13:1006777
Brown CR, Mojtahedi H, James S, Novy RG, Love S (2006) Development and evaluation of potato breeding lines with introgressed resistance to Columbia root-knot nematode (Meloidogyne chitwoodi). Am J Potato Res 83:1–8
Candido V, d’Addabbo T, Basile M, Castronuovo D, Miccolis V (2008) Greenhouse soil solarization: effect on weeds, nematodes and yield of tomato and melon. Agron Sustain Dev 28:221–230
Cap GB, Roberts PA, Thomason IJ (1993) Inheritance of heat-stable resistance to Meloidogyne incognita in Lycopersicon peruvianum and its relationship to the Mi gene. Theor Appl Genet 85:777–783
Castagnone-Sereno P (2006) Genetic variability and adaptive evolution in parthenogenetic root-knot nematodes. J Hered 96:282–289
Castagnone-Sereno P, Danchin EGJ (2014) Parasitic success without sex—the nematode experience. J Evol Biol 27:1323–1333
Castagnone-Sereno P, Mulet K, Danchin EG, Koutsovoulos GD, Karaulic M, Da Rocha MP (2019) Gene copy number variations as signatures of adaptive evolution in the parthenogenetic, plant-parasitic nematode Meloidogyne incognita. Mol Ecol 28:2559–2572
Chandrawat BS, Siddiqui AU, Bhati SS, Saharan V (2020) Response of defence related enzymes in tomato treated with oil-cakes against root-knot nematode, Meloidogyne incognita. Int J Curr Microbiol Appl Sci 9:1100–1111
Chen P, Roberts PA (2003) Virulence in Meloidogyne hapla differentiated by resistance in common bean (Phaseolus vulgaris). Nematology 5:39–47
Chen P, Tsay T (2006) Effect of crop rotation on Meloidogyne spp. and Pratylenchus spp. populations in strawberry fields in Taiwan. J Nematol 38:339
Chen T, Tibbitt CA, Feng X, Stark JM, Rohrbeck L, Rausch L, Coquet JM (2017) PPAR‑γ promotes type 2 immune responses in allergy and nematode infection. Sci Immunol 2:eaal5196
Cook R, Mizen KA, Person-Dedryver F (1999) Resistance in ryegrasses, Lolium spp., to three European populations of the root-knot nematode, Meloidogyne naasi. J Nematol 1:661–671
Coyne DL, Cortada L, Dalzell JJ, Claudius-Cole AO, Haukeland S, Luambano N, Talwana H (2018) Plant-parasitic nematodes and food security in Sub-Saharan Africa. Annu Rev Phytopathol 56:381
Curtis RH, Jones JT, Davies KG, Sharon E, Spiegel Y (2011) Plant nematode surfaces. In: Biological control of plant-parasitic nematodes. Springer, Dordrecht, pp 115–144
D’Addabbo T, Argentieri MP, Zuchowski J, Biazzi E, Tava A, Oleszek W (2020) Activity of saponins from Medicago species against phytoparasitic nematodes. Plants 9:1–19
Dash M, Somvanshi VS, Budhwar R, Godwin J, Shukla RN, Rao U (2021) A rice root-knot nematode Meloidogyne graminicola-resistant mutant rice line shows early expression of plant-defence genes. Planta 253:1–13
De Waele D, Das K, Zhao D, Tiwari RKS, Shrivastava DK, Vera-Cruz C, Kumar A (2013) Host response of rice genotypes to the rice root-knot nematode (Meloidogyne graminicola) under aerobic soil conditions. Arch Phytopathol Plant Prot 46:670–681
Decraemer W, Hunt DJ (2006) Structure and classification. In: Plant nematology, pp 3–32
Devi S, Das D (2016) Effect of organic amendments on root-knot nematode, Meloidogyne incognita in cucumber. Pest Manag Hortic Ecosyst 22:176–181
Djian-Caporalino C, Fazari A, Arguel MJ, Vernie T, VandeCasteele C, Faure I, Abad P (2007) Root-knot nematode (Meloidogyne spp.) Me resistance genes in pepper (Capsicum annuum L.) are clustered on the P9 chromosome. Theor Appl Genet 114:473–486
Dyson ZA, Seviour RJ, Tucci J, Petrovski S (2016) Genome Sequences of Pseudomonas oryzihabitans Phage POR1 and Pseudomonas aeruginosa Phage PAE1. Genome Announc 4:1515–1515
El-Deeb AM, El-Sappah AH, Arisha MH (2018) Efficiency of some bionematicides against root-knot nematode Meloidogyne incognita on three tomato cultivars under greenhouse conditions. Zagazig J Agric Res 45:2001–2010
El-Hamawi MH, Youssef MMA, Zawam HS (2004) Management of Meloidogyne incognita, the root-knot nematode, on soybean as affected by marigold and sea ambrosia (damsisa) plants. J Pest Sci 77(2):95–98
El-Sappah AH, Islam MM, El-awady H, Yan S, Qi S, Liu J, Liang Y (2019) Tomato natural resistance genes in controlling the root-knot nematode. Genes 10:925
Elling AA (2013) Major emerging problems with minor Meloidogyne species. Phytopathology 103:1092–1102
Eltayeb FME (2017) The effects of Bacillus subtilis bacteria on Meloidogyne javanica (Nematode) infection and tomato plant growth. Eur J Adv Res Bio Life Sci 6:1176–1182
Engelbrecht G, Horak I, Jansen van Rensburg PJ, Claassens S (2018) Bacillus-based bionematicides: development, modes of action and commercialization. Biocontrol Sci Technol 28:629–653
Eves-van den Akker S (2021) Plant–nematode interactions. Curr Opin Plant Biol 62:102035
Fan H, Yao M, Wang H, Zhao D, Zhu X, Wang Y, Chen L (2020) Isolation and effect of Trichoderma citrinoviride Snef1910 for the biological control of root-knot nematode, Meloidogyne incognita. BMC Microbiol 20:1–11
Food and Agricultural Organization (2010) Under nourishment around the world in 2010. In: The state of food insecurity in the world 2010
Forghani F, Hajihassani A (2020) Recent advances in the development of environmentally benign treatments to control root-knot nematodes. Front Plant Sci 11:1125
Ganaie MA, Khan TA (2010) Biological potential of Paecilomyces lilacinus on pathogenesis of Meloidogyne javanica infecting tomato plant. Eur J Appl Sci 2:80–84
Gang S, Hallem EA (2016) Mechanisms of host seeking by parasitic nematodes. Mol Biochem Parasitol 208:23–32
Gao X, Becker JO (2002) Population development of both sexes of Heterodera schachtii is diminished in a beet cyst nematode-suppressive soil. Biol Control 25:187–194
Gao B, Allen R, Davis EL, Baum TJ, Hussey RS (2004) Molecular characterization and developmental expression of a cellulose-binding protein gene in the soybean cyst nematode Heterodera glycines. Int J Parasitol 34:1377–1383
Gheysen G, Mitchum MG (2011) How nematodes manipulate plant development pathways for infection. Curr Opin Plant Biol 14:415–421
Giannakou IO, Anastasiadis IA, Gowen SR, Prophetou-Athanasiadou DA (2007) Effects of a non-chemical nematicide combined with soil solarization for the control of root-knot nematodes. Crop Prot 26:1644–1654
Gleason C, Polzin F, Habash SS, Zhang L, Utermark J, Grundler FM, Elashry A (2017) Identification of two Meloidogyne hapla genes and an investigation of their roles in the plant-nematode interaction. Mol Plant Microbe Interact 30:101–112
Gopal M, Gupta A, Thomas GV (2012) Vermicompost and vermiwash add beneficial micro flora that enhance soil quality and sustain crop growth. Int J Innov Hortic 1(2):93–100
Goverse A, Smant G (2014) The activation and suppression of plant innate immunity by parasitic nematodes. Ann Rev Phytopathol 52:243–265
Haegeman A, Mantelin S, Jones JT, Gheysen G (2012) Functional roles of effectors of plant-parasitic nematodes. Gene 492:19–31
Hallmann J, Davies KG, Sikora R (2009) Biological control using microbial pathogens, endophytes and antagonist. In: Root-knot nematodes, p 380
Haque Z, Khan MR (2022) Organic management of rice root-knot nematode, Meloidogyne graminicola. In: Organic Management. Sustainable management of nematodes in agriculture, vol 1. Springer, Cham, pp 247–267
Haroon SA (2018) Genetic fingerprinting of root knot nematode as important pest in EGYPT. Ann Agric Sci Moshtohor 56(4th ICBAA):17–22
Hassanin AA, Saad AM, Bardisi EA, Salama A, Sitohy MZ (2020) Transfer of anthocyanin accumulating delila and rosea1 genes from the transgenic tomato micro-tom cultivar to moneymaker cultivar by conventional breeding. J Agric Food Chem 68:10741–10749
Hewezi T (2015) Cellular signaling pathways and posttranslational modifications mediated by nematode effector proteins. Plant Physiol 169:1018–1026
Hewezi T, Baum TJ (2013) Manipulation of plant cells by cyst and root-knot nematode effectors. Mol Plant-Microbe Interact 26:9–16
Ho JY, Weide R, Ma HM, Van Wordragen MF, Lambert KN, Koornneef M, Williamson VM (1992) The root-knot nematode resistance gene (Mi) in tomato: construction of a molecular linkage map and identification of dominant cDNA markers in resistant genotypes. Plant J 2:971–982
Hu L, Cui R, Sun L, Lin B, Zhuo K, Liao J (2013) Molecular and biochemical characterization of the β‑1, 4‑endoglucanase gene Mj-eng‑3 in the root-knot nematode Meloidogyne javanica. Exp Parasitol 135:15–23
Hu Y, Li J, Li J, Zhang F, Wang J, Mo M, Liu Y (2019) Biocontrol efficacy of Pseudoxanthomonas japonensis against Meloidogyne incognita and its nematostatic metabolites. FEMS Microbiol Lett 366:fny287
Huang WK, Sun JH, Cui JK, Wang GF, Kong LA, Peng H, Peng DL (2014) Efficacy evaluation of fungus Syncephalastrum racemosum and nematicide avermectin against the root-knot nematode Meloidogyne incognita on cucumber. PLoS ONE 9:89717
Hussain M, Hamid MI, Tian J, Hu J, Zhang X, Chen J, Liu X (2018) Bacterial community assemblages in the rhizosphere soil, root endosphere and cyst of soybean cyst nematode-suppressive soil challenged with nematodes. FEMS Microbiol Ecol 94:fiy142
Hussain T, Haris M, Shakeel A, Ahmad G, Ahmad Khan A, Khan M (2020) Bio-nematicidal activities by culture filtrate of Bacillus subtilis Hussain T‑AMU: New promising biosurfactant bioagent for the management of Root Galling caused by Meloidogyne incognita. Vegetos 33:229–238
Hussey RS, Janssen GJW (2002) Root-knot nematodes: Meloidogyne species. In: Plant resistance to parasitic nematodes. Cabi, Wallingford, pp 43–70
Iberkleid I, Vieira P, de Almeida Engler J, Firester K, Spiegel Y, Horowitz SB (2013) Fatty acid-and retinol-binding protein, Mj-FAR‑1 induces tomato host susceptibility to root-knot nematodes. PLoS ONE 8:e64586
Jagdale S, Rao U, Giri AP (2021) Effectors of root-knot nematodes: an arsenal for successful parasitism. Front Plant Sci 12:800030
Jaouannet M, Rosso MN (2013) Effectors of root sedentary nematodes target diverse plant cell compartments to manipulate plant functions and promote infection. Plant Signal Behav 8:25507
Jaouannet M, Magliano M, Arguel MJ, Gourgues M, Evangelisti E, Abad P, Rosso MN (2013) The root-knot nematode calreticulin Mi-CRT is a key effector in plant defense suppression. Mol Plant-Microbe Interact 26(1):97–105
Javeed MT, Al-Hazmi AS, Molan YY (2016) Antagonistic effects of some indigenous isolates of Trichoderma spp. against Meloidogyne javanica. Pak J Nematol 34:183–191
Jena P, Sahoo NK, Mahalik JK (2021) Effect of oil cakes and bio-agents each alone and in combination for management of root knot nematode (Meloidogyne incognita) in green gram. Int J Bioresour Stress Manag 12:286–294
Ji X, Li J, Meng Z, Dong S, Zhang S, Qiao K (2019) Inhibitory effect of allicin against Meloidogyne incognita and Botrytis cinerea in tomato. Sci Hortic 253:203–208
Jones JD, Dangl JL (2006) The plant immune system. Nature 444:323–329
Kankam F, Sowley ENK (2016) Evaluation of neem (Azadirachtaindica L.) products for the control of root-knot nematode of chilli pepper (Capsicum annum L.). Arch Phytopathol Plant Prot 49:111–119
Kankam F, Sowley ENK, Dankwa IN (2014) Management of root knot nematode (Meloidogyne incognita) on cowpea (Vigna unguiculata L. Walp.) with oil cakes. Int J Biosci 5:413–419
Kankam F, Sowley ENK, Mohammed A (2015) Management of root-knot nematode (Meloidogyne spp.) on okra (Abelmoschus esculentus L. Moench) with aqueous sesame seed extract. Int J Agron Agric Res 6:24–31
Katan J, Greenberger A, Alon H, Grinstein A (1976) Solar heating by polyethylene mulching for the control of diseases caused by soil-borne pathogens. Phytopathology 66:683–688
Khalil MSEDH, Allam AFG, Barakat AST (2012) Nematicidal activity of some biopesticide agents and microorganisms against root-knot nematode on tomato plants under greenhouse conditions. J Plant Prot Res. https://doi.org/10.2478/v10045-012-0008-5
Khan A, Williams KL, Nevalainen HK (2006) Control of plant-parasitic nematodes by Paecilomyces lilacinus and Monacrosporium lysipagum in pot trials. Biocontrol 51:643–658
Khan A, Shaukat SS, Sayed M (2011) Control of nematodes associated with almond using oil-cakes in Balochistan. Pak J Nematol 29:171–177
Khan A, Ahmad G, Haris M, Khan AA (2022a) Bio-organics management: novel strategies to manage root-knot nematode, Meloidogyne incognita pest of vegetable crops. Gesunde Pflanz 75:193–209
Khan A, Ansari MSA, Irsad, Hussain T, Khan AA (2022b) Role of beneficial microbes for plant growth improvement. In: Plant protection: from chemicals to biologicals, p 141
Khan A, Khan F, Shariq M, Asif M, Ansari T, Fatima S, Siddiqui MA (2022c) Screening for resistance and susceptibility in some beetroot cultivars to root-knot nematode, Meloidogyne javanica. J Environ Biol 43:571–577
Khan AR, Javed N, Sahi ST, Mukhtar T, Khan SA, Ashraf W (2017) Glomus mosseae (Gerd & Trappe) and neemex reduce invasion and development of Meloidogyne incognita. Pak J Zool 49:841–847
Khan F, Asif M, Khan A, Tariq M, Siddiqui MA (2018) Screening of carrot cultivars against root-knot nematode Meloidogyne incognita. Indian Phytopathol 71:415–421
Khan F, Asif M, Khan A, Tariq M, Ansari T, Shariq M, Siddiqui MA (2019) Evaluation of the nematicidal potential of some botanicals against root-knot nematode, Meloidogyne incognita infected carrot: In vitro and greenhouse study. Curr Plant Biol 20:100115
Khan F, Shariq M, Asif M, Ansari T, Fatima S, Khan A, Siddiqui MA (2022d) Organic nematicides: a green technique and its overview for nematode pest management. In: Organic management. Sustainable management of nematodes in agriculture, vol 1. Springer, Cham, pp 39–66
Khan MR, Jain RK, Ghule TM, Pal S (2014) Root knot nematodes in India. A comprehensive monograph. All India co-ordinated research project on plant parasitic nematodes with integrated approach for their control. Indian Agricultural Research Institute, New Delhi
Khan RAA, Najeeb S, Mao Z, Ling J, Yang Y, Li Y, Xie B (2020) Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic bacteria and root-knot nematode. Microorganisms 8:401
Kihika R, Murungi LK, Coyne D, Ng’ang’a M, Hassanali A, Teal PE, Torto B (2017) Parasitic nematode Meloidogyne incognita interactions with different Capsicum annum cultivars reveal the chemical constituents modulating root herbivory. Sci Rep 7:1–10
Kim J, Yang R, Chang C, Park Y, Tucker ML (2018) The root-knot nematode Meloidogyne incognita produces a functional mimic of the Arabidopsis inflorescence deficient in abscission signaling peptide. J Exp Bot 69:3009–3021
Klocke E, Nothnagel T, Schumann G (2010) Vegetables. In: Genet Mod Plant, pp 499–550
Kokalis-Burelle N (2015) Pasteuria penetrans for control of Meloidogyne incognita on tomato and cucumber, and M. arenaria on snapdragon. J Nematol 47:207
Kokalis-Burelle N, Rosskopf EN, Butler DM, Fennimore SA, Holzinger J (2016) Evaluation of steam and soil solarization for Meloidogyne arenaria control in Florida floriculture crops. J Nematol 48:183–192
Kudla U, Qin L, Milac A, Kielak A, Maissen C, Overmars H, Helder J (2005) Origin, distribution and 3D-modeling of Gr-EXPB1, an expansin from the potato cyst nematode Globodera rostochiensis. FEBS Lett 579:2451–2457
Kumar M (2017) Efficacy of different oil–cakes on the incidence of root–knot nematodes (Meloidogyne javanica) on tomato. J Middle East North Afr Sci 3:41–43
Kumar S, Khanna AS (2008) Role of Trichoderma harzianum and neem cake separately and in combination against root-knot nematode on tomato. Indian J Nematol 38:264–266
Laquale S, Candido V, Avato P, Argentieri MP, d’Addabbo T (2015) Essential oils as soil biofumigants for the control of the root-knot nematode Meloidogyne incognita on tomato. Ann Appl Biol 167:217–224
Lee YS, Kim KY (2016) Antagonistic potential of Bacillus pumilus L1 against root-knot nematode, Meloidogyne arenaria. J Phytopathol 164:29–39
Leontopoulos SV, Petrotos K, Anatolioti V, Skenderidis P, Tsilfoglou S, Vagelas I (2017) Chemotactic responses of Pseudomonas oryzihabitans and second stage juveniles of Meloidogyne javanica on tomato root tip exudates. Int J Food Biosyst Eng 5:75–100
Li J, Zou C, Xu J, Ji X, Niu X, Yang J, Zhang KQ (2015) Molecular mechanisms of nematode-nematophagous microbe interactions: basis for biological control of plant-parasitic nematodes. Annu Rev Phytopathol 53:67–95
Li S, Duan Y, Zhu X, Chen L, Wang Y, Pan L (2011) Effects of adding secondary metabolites of Aspergillus niger on resistance to tomato root-knot nematode. China Veg 4:44–49
Lima FS, Correa VR, Nogueira SR, Santos PR (2017) Nematodes affecting soybean and sustainable practices for their management. In: Soybean–basis of yield, biomass and productivity, pp 95–110
Lin B, Zhuo K, Chen S, Hu L, Sun L, Wang X, Liao J (2016) A novel nematode effector suppresses plant immunity by activating host reactive oxygen species-scavenging system. New Phytol 209:1159–1173
Liu G, Lin X, Xu S, Liu G, Liu F, Mu W (2020) Screening, identification and application of soil bacteria with nematicidal activity against root-knot nematode (Meloidogyne incognita) on tomato. Pest Manag Sci 76:2217–2224
Lopes EA, Dallemole-Giaretta R, Santos Neves WD, Parreira DF, Ferreira PA (2019) Eco-friendly approaches to the management of plant-parasitic nematodes. In: Plant health under biotic stress. Springer, Singapore, pp 167–186
Lopez-Llorca LV, Claugher D (1990) Appressoria of the nematophagous fungus Verticillium suchlasporium. Micron Microsc Acta 21:125–130
Machado ACZ (2014) Current nematode threats to Brazilian agriculture. Curr Agric Sci Technol 20(1):26–35
Mauromicale G, Lo Monaco A, Longo AMG (2010) Improved efficiency of soil solarization for growth and yield of greenhouse tomatoes. Agron Sustain Dev 30(4):753–761
Mejias J, Bazin J, Truong NM, Chen Y, Marteu N, Bouteiller N, Quentin M (2021) The root-knot nematode effector MiEFF18 interacts with the plant core spliceosomal protein SmD1 required for giant cell formation. New Phytol 229:3408–3423
Melero-Vara JM, Lopez-Herrera CJ, Basallote-Ureba MJ, Prados AM, Vela MD, Macias FJ, Talavera M (2012) Use of poultry manure combined with soil solarization as a control method for Meloidogyne incognita in carnation. Plant Dis 96:990–996
Mendoza AR, Kiewnick S, Sikora RA (2008) In vitro activity of Bacillus firmus against the burrowing nematode Radopholus similis, the root-knot nematode Meloidogyne incognita and the stem nematode Ditylenchus dipsaci. Biocontrol Sci Technol 18:377–389
Metwally WE, Mostafa FAM, Refaei AR (2015) In vitro study on the antagonistic activity of different native isolates of rhizobacteria against Meloidogyne incognita. Egypt J Agronematol 14:1–9
Mitiku M (2018) Plant-parasitic nematodes and their management: A review. Agric Res Technol 8:30–38
Mnif I, Ghribi D (2015) Review: Potential of bacterial derived biopesticides in pest management. Crop Prot 77:52–64
Moens M, Perry RN (2009) Migratory plant endoparasitic nematodes: a group rich in contrasts and divergence. Annu Rev Phytopathol 47:313–332
Mukhtar T (2018) Management of root-knot nematode, Meloidogyne incognita, in tomato with two Trichoderma species. Pak J Zool 50:1589–1592
Naalden D, Haegeman A, de Almeida-Engler J, Birhane-Eshetu F, Bauters L, Gheysen G (2018) The Meloidogyne graminicola effector Mg16820 is secreted in the apoplast and cytoplasm to suppress plant host defense responses. Mol Plant Pathol 19:2416–2430
Naz I, Khan RAA, Masood T, Baig A, Siddique I, Haq S (2021) Biological control of root knot nematode, Meloidogyne incognita, in vitro, greenhouse and field in cucumber. Biocontrol 152:104429
Nguyen CN, Perfus-Barbeoch L, Quentin M, Zhao J, Magliano M, Marteu N, Favery B (2018) A root-knot nematode small glycine and cysteine-rich secreted effector, MiSGCR1, is involved in plant parasitism. New Phytol 217:687–699
Nico AI, Jiménez-Díaz RM, Castillo P (2003) Solarization of soil in piles for the control of Meloidogyne incognita in olive nurseries in southern Spain. Plant Pathol 52:770–778
Niu J, Liu P, Liu Q, Chen C, Guo Q, Yin J, Jian H (2016) Msp40 effector of root-knot nematode manipulates plant immunity to facilitate parasitism. Sci Rep 6:1–13
Ntalli N, Monokrousos N, Rumbos C, Kontea D, Zioga D, Argyropoulou MD, Tsiropoulos NG (2018) Greenhouse bio fumigation with Melia azedarach controls Meloidogyne spp. and enhances soil biological activity. J Pest Sci 91:29–40
Ntalli N, Bratidou-Parlapani A, Tzani K, Samara M, Boutsis G, Dimou M, Monokrousos N (2020) Thymus citriodorus (Schreb) botanical products as eco-friendly nematicides with bio-fertilizing properties. Plants 9:202
Nyczepir AP, Thomas SH (2009) 18 current and future management strategies in intensive crop production systems. In: Root-knot nematodes, p 412
Olabiyi TI, Ayeni BP (2016) Assessment of Azadirachta indica and Cleome viscosa liquid-formulations as bio-nematicides in the management of nematode pests of okra. Afr J Agric Res 11:467–471
Olufolaji DB, Ajayi AM (2021) Ecofriendly management of plant diseases in Sub-Saharan Africa. Indian J Phytopathol 74:477–484
Oluwatayo JI, Jidere CI, Nwankiti A (2019) Nematicidal effect of some botanical extracts for the management of Meloidogyne incognita and on growth of tomato. Asian J Agric Hortic Res 4:1–8
Opperman CH, Bird DM, Williamson VM, Rokhsar DS, Burke M, Cohn J, Windham E (2008) Sequence and genetic map of Meloidogyne hapla: A compact nematode genome for plant parasitism. Proc Natl Acad Sci 105:14802–14807
Ornat C, Verdejo-Lucas S, Sorribas FJ (2001) A population of Meloidogyne javanica in Spain virulent to the Mi resistance gene in tomato. Plant Dis 85:271–276
Palomares-Rius JE, Escobar C, Cabrera J, Vovlas A, Castillo P (2017) Anatomical alterations in plant tissues induced by plant-parasitic nematodes. Front Plant Sci 8:1987
Parihar K, Rehman B, Ganai MA, Asif M, Siddiqui MA (2015) Role of Oil Cakes and Pochonia chlamydosporia for the Management of Meloidogyne javanica attacking Solanum melongena L. Int J Plant Path 6:12–20
Patidar RK, Sen D, Pathak M, Shakywar RC (2016) Effect of essential oils on mortality, hatching and multiplication of root-knot nematode, Meloidogyne incognita and its Impact on plant growth parameters. Int J Agric Environ Biotechnol 9:887–895
Peiris PUS, Li Y, Brown P, Xu C (2020) Fungal biocontrol against Meloidogyne spp. in agricultural crops: A systematic review and meta-analysis. Biocontrol 144:104235
Petitot AS, Kyndt T, Haidar R, Dereeper A, Collin M, de Almeida Engler J, Fernandez D (2017) Transcriptomic and histological responses of African rice (Oryza glaberrima) to Meloidogyne graminicola provide new insights into root-knot nematode resistance in monocots. Ann Bot 119:885–899
Qin L, Kudla U, Roze EH, Goverse A, Popeijus H, Nieuwland J, Helder J (2004) A nematode expansin acting on plants. Nature 427:30–30
Qin X, Xue B, Tian H, Fang C, Yu J, Chen C, Wang X (2022) An unconventionally secreted effector from the root knot nematode Meloidogyne incognita, Mi-ISC‑1, promotes parasitism by disrupting salicylic acid biosynthesis in host plants. Mol Plant Pathol 23:516–529
Quentin M, Abad P, Favery B (2013) Plant parasitic nematode effectors target host defense and nuclear functions to establish feeding cells. Front Plant Sci 4:53
Radwan MA, El-Maadawy EK, Kassem SI, Abu-Elamayem MM (2009) Oil cakes soil amendment effects on Meloidogyne incognita, root-knot nematode infecting tomato. Arch Phytopathol Plant Prot 42:58–64
Ramezani Moghaddam M, Mahdikhani Moghaddam E, Baghaee Ravari S, Rouhani H (2014) The nematicidal potential of local Bacillus species against the root-knot nematode infecting greenhouse tomatoes. Biocontrol Sci Technol 24(3):279–290
Ravindra H, Sehgal M, Narasimhamurthy HB, Jayalakshmi K, Khan I (2017) Rice root-knot nematode (Meloidogyne graminicola) an emerging problem. Int J Curr Microbiol App Sci 6:3143–3171
Reddy PP, Reddy PP (2021) Tropical fruit crops. In: Nematode diseases of crops and their management, pp 147–188
Rehman B, Ganai MA, Parihar K, Siddiqui MA, Usman A (2012) Management of root-knot nematode, Meloidogyne incognita affecting chickpea, Cicer arietinum for sustainable production. Biosci Int 1:1–5
Rehman B, Ganai MA, Parihar K, Asif M, Siddiqui MA (2015) Biopotency of oil cakes against Meloidogyne incognita affecting Vigna mungo. Asian J Crop Sci 7:128–137
Sansinenea E, Ortiz A (2011) Secondary metabolites of soil Bacillus spp. Biotechnol Lett 33:1523–1538
Santra HK, Banerjee D (2020) Fungal endophytes: a source for biological control agents. In: Agriculturally important fungi for sustainable agriculture. Springer, Cham, pp 181–216
Shahzaman S, Inam-ul-Haq M, Mukhtar T, Naeem M (2015) Isolation, identification of antagonistic rhizo-bacterial strains obtained from chickpea (Cicer arietinum L.) field and their in-vitro evaluation against fungal root pathogens. Pak J Bot 47:1553–1558
Shi Q, Mao Z, Zhang X, Ling J, Lin R, Zhang X, Xie B (2018) The novel secreted Meloidogyne incognita effector MiISE6 targets the host nucleus and facilitates parasitism in Arabidopsis. Front Plant Sci 9:252
Shilpa, Sharma P, Thakur V, Sharma A, Rana RS, Kumar P (2022) A status-quo review on management of root knot nematode in tomato. J Hortic Sci Biotechnol 97(4):403–416
Sikandar A, Zhang M, Wang Y, Zhu X, Liu X, Fan H, Duan Y (2020) Nematodes a risk to agriculture. Appl Ecol Environ Res 18:1679–1690
Sikora RA, Fernandez E (2005) Nematode parasites of vegetables. In: Plant parasitic nematodes in subtropical and tropical agriculture. CABI, Wallingford, pp 319–392
Silva MF, Campos VP, Barros AF, Terra WC, Pedroso MP, Gomes VA, Silva FJ (2020) Volatile emissions of watercress (Nasturtium officinale) leaves and passion fruit (Passiflora edulis) seeds against Meloidogyne incognita. Pest Manag Sci 76:1413–1421
Silva SD, Carneiro RM, Faria M, Souza DA, Monnerat RG, Lopes RB (2017) Evaluation of Pochonia chlamydosporia and Purpureocillium lilacinum for suppression of Meloidogyne enterolobii on tomato and banana. J Nematol 49:77–85
Singh T, Patel BA (2015) Management of root-knot nematode (Meloidogyne incognita) in bottle gourd using different botanicals in pots. J Parasit Dis 39:441–445
Singh S, Singh B, Singh AP (2015) Nematodes: a threat to sustainability of agriculture. Proc Environ Sci 29:215–216
Smant G, Helder J, Goverse A (2018) Parallel adaptations and common host cell responses enabling feeding of obligate and facultative plant parasitic nematodes. Plant J 93:686–702
Soliman MS, El-Deriny MM, Ibrahim DSS, Zakaria H, Ahmed Y (2021) Suppression of root-knot nematode Meloidogyne incognita on tomato plants using the nematode trapping fungus Arthrobotrys oligospora Fresenius. J App Microbiol 131:2402–2415
Starr JL, Mercer CF (2009) Development of resistant varieties. In: Perry RN, Moens M, Starr JL (eds) Root-knot nematodes. CAB International, Wallingford, pp 326–337
Stirling GR, West LM (1991) Fungal parasites of root-knot nematode eggs from tropical and subtropical regions of Australia. Aust Plant Pathol 20:149–154
Tapia-Vazquez I, Montoya-Martínez AC, los Santos-Villalobos D, Ek-Ramos MJ, Montesinos-Matías R, Martínez-Anaya C (2022) Root-knot nematodes (Meloidogyne spp.) a threat to agriculture in Mexico: Biology, current control strategies, and perspectives. World J Microbiol Biotechnol 38:1–18
Tariq M, Ameen F, Khan A, Alkahtani MD, Siddiqui MA (2022) Repellent and nematostatic behaviour of botanical extracts against root-knot nematode Meloidogyne incognita attacking Solanum melongena L. Pol J Environ Stud 31(1):307–314
Teillet A, Dybal K, Kerry BR, Miller AJ, Curtis RH, Hedden P (2013) Transcriptional changes of the root-knot nematode Meloidogyne incognita in response to Arabidopsis thaliana root signals. PLoS ONE 8:61259
Thoden TC, Korthals GW, Termorshuizen AJ (2011) Organic amendments and their influences on plant-parasitic and free-living nematodes: a promising method for nematode management. J Nematol 13:133–153
Tibugari H, Mombeshora D, Mandumbu R, Karavina C, Parwada C (2012) A comparison of the effectiveness of the aqueous extracts of garlic, castor beans and marigold in the biocontrol of root-knot nematode in tomato. J Agric Technol 8(2):479–492
Tiwari S, Pandey S, Singh Chauhan P, Pandey R (2017) Biocontrol agents in co-inoculation manages root knot nematode [Meloidogyne incognita (Kofoid & White) chitwood] and enhances essential oil content in Ocimum basilicum L. J Ind Crop Prod 97:292–301
Tiyagi SA, Ajaz S (2004) Biological control of plant-parasitic nematodes associated with chickpea using oil cakes and Paecilomyces lilacinus. Ind J Nematol 34:44–48
Truong NM, Chen Y, Mejias J, Soule S, Mulet K, Jaouannet M, Quentin M (2021) The Meloidogyne incognita nuclear effector MiEFF1 interacts with Arabidopsis cytosolic glyceraldehyde-3-phosphate dehydrogenases to promote parasitism. Front Plant Sci 12:641480
Turatto MF, Dourado FDS, Zilli JE, Botelho GR (2018) Control potential of Meloidogyne javanica and Ditylenchus spp. using fluorescent Pseudomonas and Bacillus spp. Braz J Microbiol 49:54–59
Tzortzakakis EA, Adam MA, Blok VC, Paraskevopoulos C, Bourtzis K (2005) Occurrence of resistance-breaking populations of root-knot nematodes on tomato in Greece. Eur J Plant Pathol 113:101–105
Vaitheeswaran M, Mohamad IS, Lakshmi NK (2005) Organic amendments for the control of root-knot nematode on Phaseolus mungo. Indian J Nematol 35:112–119
Van Den Hoogen J, Geisen S, Routh D, Ferris H, Traunspurger W, Wardle DA, Crowther TW (2019) Soil nematode abundance and functional group composition at a global scale. Nature 572:194–198
Verdejo-Lucas S, Sorribas F (2008) Resistance response of the tomato rootstock SC 6301 to Meloidogyne javanica in a plastic house. Eur J Plant Pathol 121:103–107
Vianene NM, Abawi GS (2000) Hirsutella rhossiliensis and Verticillium chlamydosporium as Biocontrol agents of the root-knot nematode Meloidogyne hapla on lettuce. J Nematol 32:85
Vieira P, Gleason C (2019) Plant-parasitic nematode effectors-insights into their diversity and new tools for their identification. Curr Opin Plant Biol 50:37–43
Vieira P, Danchin EG, Neveu C, Crozat C, Jaubert S, Hussey RS, Rosso MN (2011) The plant apoplasm is an important recipient compartment for nematode secreted proteins. J Exp Bot 62:1241–1253
Viljoen JJ, Labuschagne N, Fourie H, Sikora RA (2019) Biological control of the root-knot nematode Meloidogyne incognita on tomatoes and carrots by plant growth-promoting rhizobacteria. Trop Plant Pathol 44:284–291
Wang C, Bruening G, Williamson VM (2009) Determination of preferred pH for root-knot nematode aggregation using pluronic F‑127 gel. J Chem Ecol 35:1242–1251
Wang JY, Guo C, Zhao P, Yu FY, Su Y, Qu JP, Zhou B (2021) Biocontrol potential of Bacillus altitudinis AMCC1040 against root-knot nematode disease of ginger and its impact on rhizosphere microbial community. Biocontrol 158:104598
Wang XR, Moreno YA, Wu HR, Ma C, Li YF, Zhang JA, Geary TG (2012) Proteomic profiles of soluble proteins from the esophageal gland in female Meloidogyne incognita. Int J Parasitol 42:1177–1183
Wen Y, Meyer SL, MacDonald MH, Zheng L, Jing C, Chitwood DJ (2019) Nematotoxicity of Paeonia spp. extracts and Camellia oleifera tea seed cake and extracts to Heterodera glycines and Meloidogyne incognita. Plant Dis 103:2191–2198
Wesemael WML, Moens M (2012) Screening of common bean (Phaseolus vulgaris) for resistance against temperate root-knot nematodes (Meloidogyne spp.). Pest Manag Sci 68:702–708
Westerdahl BB (2018) Evaluation of trap cropping for management of root-knot nematode on annual crops. In: IX International Symposium on Soil and Substrate Disinfestation 1270, pp 141–146
Wondimeneh T, Sakhuja PK, Tadele T (2013) Root-knot nematode (Meloidogyne incognita) management using botanicals in tomato (Lycopersicon esculentum). Acad J Agri Res 1:9–16
Xiang N, Lawrence KS, Kloepper JW, Donald PA, McInroy JA, Lawrence GW (2017) Biological control of Meloidogyne incognita by spore-forming plant growth-promoting rhizobacteria on cotton. Plant Dis 101:774–784
Xie J, Li S, Mo C, Wang G, Xiao X, Xiao Y (2016) A novel Meloidogyne incognita effector Misp12 suppresses plant defense response at latter stages of nematode parasitism. Front Plant Sci 7:964
Yang XJ, Wang X, Wang K, Su LX, Li HM, Li R, Shen QR (2015) The Nematicidal effect of camellia seed cake on root-knot nematode, Meloidogyne javanica of banana. PLoS ONE 10:119700
Youssef MMA, El-Nagdi WM (2004) Cellular alteration of root-knot nematode, Meloidogyne incognita infected squash plant and intercropping sesame plant or sesame oil seed cake as control measures. Egypt J Phytopath 32:77–85
Yuhui B, Gao C, Yu Z (2018) Rhabdopeptides from Xenorhabdus budapestensis SN84 and their nematicidal activities against Meloidogyne incognita. J Agric Food Chem 66:3833–3839
Zhang L, Gleason C (2020) Enhancing potato resistance against root-knot nematodes using a plant-defense elicitor delivered by bacteria. Nat Plants 6:625–629
Zhao D, Zhao H, Zhao D, Zhu X, Wang Y, Duan Y, Chen L (2018) Isolation and identification of bacteria from rhizosphere soil and their effect on plant growth promotion and root-knot nematode disease. Biocontrol 119:12–19
Zhao J, Wang S, Zhu X, Wang Y, Liu X, Duan Y, Chen L (2021) Isolation and characterization of nodules endophytic bacteria Pseudomonas protegens Sneb1997 and Serratia plymuthica Sneb2001 for the biological control of root-knot nematode. Appl Soil Ecol 164:103924
Zhou L, Yuen G, Wang Y, Wei L, Ji G (2016) Evaluation of bacterial biological control agents for control of root-knot nematode disease on tomato. Crop Prot 84:8–13
Zhou Y, Zhao D, Shuang L, Xiao D, Xuan Y, Duan Y, Zhu X (2020) Transcriptome analysis of rice roots in response to root-knot nematode infection. Int J Mol Sci 21:848
Zhuo K, Chen J, Lin B, Wang J, Sun F, Hu L, Liao J (2017) A novel Meloidogyne enterolobii effector MeTCTP promotes parasitism by suppressing programmed cell death in host plants. Mol Plant Pathol 18:45–54
Author information
Authors and Affiliations
Contributions
A. Khan and M.A. Siddiqui: Designed the manuscript; A. Khan and A. Khan: Wrote the manuscript; A. Ali and F. Saba: Prepared figures and tables, M.A. Siddiqui: Supervised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
A. Khan, A. Khan, A. Ali, S. Fatima and M.A. Siddiqui declare that they have no competing interests.
Rights and permissions
Springer Nature oder sein Lizenzgeber (z.B. eine Gesellschaft oder ein*e andere*r Vertragspartner*in) hält die ausschließlichen Nutzungsrechte an diesem Artikel kraft eines Verlagsvertrags mit dem/den Autor*in(nen) oder anderen Rechteinhaber*in(nen); die Selbstarchivierung der akzeptierten Manuskriptversion dieses Artikels durch Autor*in(nen) unterliegt ausschließlich den Bedingungen dieses Verlagsvertrags und dem geltenden Recht.
About this article
Cite this article
Khan, A., Khan, A., Ali, A. et al. Root-Knot Nematodes (Meloidogyne spp.): Biology, Plant-Nematode Interactions and Their Environmentally Benign Management Strategies. Gesunde Pflanzen 75, 2187–2205 (2023). https://doi.org/10.1007/s10343-023-00886-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10343-023-00886-5