Abstract
The substantial impact of cotton insects and the lack of effective control strategies are the main limiting factors for cotton production. In greenhouse experiments, the vertical and horizontal distribution of Helicoverpa armigera (Hübner) eggs was manipulated. Trichogramma minutum (Riley) and T. pretiosum (Riley) differed significantly in searching behaviour as measured by parasitization rates on three cotton cultivars. Parasitization rates were higher on the upper and lower leaves than on the middle leaves. Furthermore, parasitization rates were negatively correlated with distance between the releasing site and egg batches on the cotton plants. Morphological traits, i.e. presence of black glands or trichome densities of the cotton cultivars played a significant role. The parasitization rates on cultivars with glands and lower trichome density were higher than with no glands and high trichome density. Moreover, GC-MS analysis revealed that volatiles and the phytosterol composition of leaves were significantly different for cotton cultivars. These chemical traits of host plants are considered in relation to Trichogramma behaviour.
Zusammenfassung
Baumwolle wird von einem großen Insektenspektrum befallen. Die größten Schäden werden dabei von Helicoverpa armigera, einer Schmetterlingsart, verursacht. Biologische Maßnahmen für die Kontrolle der Schädlingsarten sind bisher jedoch in den meisten Fällen noch nicht etabliert. Daher wurde diese Studie mit dem Ziel durchgeführt, die Wirksamkeit verschiedener biologischer Kontrollmaßnahmen gegenüber H. armigera in einer organischen Baumwollproduktion zu überprüfen. Folgende Fragestellung stand im Mittelpunkt: Welchen Einfluss haben die chemischen und morphologischen Merkmale verschiedener Baumwollsorten auf das Suchverhalten und die Parasitierungsrate der Trichogramma-Arten?
Trichogramma minutum und T. pretiosum unterscheiden sich erheblich in ihrem Erfolg, vertikal verteilte Eier zu finden. Die Parasitisierungsraten waren auf den oberen und unteren Blattetagen verschiedener Baumwollsorten höher als auf den mittleren Blättern. Außerdem waren die Parasitisierungsraten negativ mit der Distanz zwischen dem Ort der Freisetzung der Parasitoide und dem Ort der Eierposition korreliert. Morphologische Merkmale der Baumwollpflanzen, dass heißt, das Vorhandensein von Drüsen oder die Dichte von Trichomen, beeinflussten das Verhalten von Trichogramma signifikant. Die Parasitisierungsrate auf Sorten mit Blattdrüsen und verringerter Trichomdichte war signifikant höher als auf Sorten ohne Blattdrüsen und hoher Trichomdichte. Eine Analyse der volatilen Substanzen mittels GC-MS zeigte, dass sich die Baumwollsorten erheblich, sowohl bei diesen Substanzen, als auch in der Zusammensetzung ihrer Phytosterole unterschieden.
Die Resultate dieser Experimente werden hinsichtlich der Nachfrage nach biologischen Kontrollstrategien in organisch angebauter Baumwolle diskutiert. Großflächige Feldexperimente sind nötig, um abschätzen zu können, ob diese Maßnahmen auch im Freiland zur Anwendung kommen können.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trichogramma are the largest and most widely used genus (ca. 200 species) of natural enemies used in biological control of lepidopteran pests (Smith 1996; Romeis et al. 1997). Although Trichogramma have attracted considerable attention for over a century and they play a significant role in the biological control of insect pests, there is only limited information regarding the influence of plant physical and chemical traits on their behaviour. Several plant characters have been presumed to affect the searching behaviour and efficiency of Trichogramma egg parasitoids (Romeis et al. 1998, 2005).
Most research on carnivore foraging behaviour has considered rewarding experiences such as the finding of a host or a host’s products (Lukianchuk and Smith 1997; Vet et al. 1998; Gingras and Boivin 2002). Previous studies on the effect of physical characteristics on behaviour of parasitoids have focused on a single component, i.e. plant size (Thorpe 1985; Romeis et al. 1998). Ables et al. (1980) recognized three components of plant structure (e.g. trichome) and studied their effects on searching behaviour of parasitoids (Gingras et al. 2003, 2008). Successful parasitism by parasitoids is preceded by several phases of host searching that lead females into the close vicinity of their potential host (Gingras et al. 2002; Grieshop et al. 2007).
The chemical attributes of host plants may serve as signals for the parasitoids to locate host eggs (Vet and Dicke 1992). Phytosterols are the integral component of plant cell membranes and are indispensable nutrition for herbivorous insects (Bilger et al. 1997). Chemical stimuli emitted by herbivorous host or the host’s food plant are often used by parasitoids and predators in the host-searching process (De Moraes et al. 1998; Dicke 1999; Runyon et al. 2008). Jasmonic acid is found in many plants and is involved in regulating diverse plant functions, including plant resistance (Creelman and Mullet 1997). Plant volatiles can also serve as a chemical defense by recruiting beneficial insects that are natural enemies of the herbivore, thereby providing an indirect protection to the plant (Turlings et al. 1995; Kessler and Baldwin 2001).
Arthropods are well known for a well-developed sense of smell (Mustaparta 1984). Carnivores can discriminate between plants of different species that are infested by herbivores of the same species (Takabayashi et al. 1994). Volatiles emitted from jasmonate application or larval damage serve as essential host-location cues for parasitic insects of several agricultural pest species (Thaler et al. 1996; Paré and Tumlinson 1998, 1999; Rodriguez-Saona et al. 2001). By directly comparing volatile emission patterns of glanded cotton plants treated with jasmonate or damaged by herbivores, compounds induced by jasmonate can be distinguished from those that are emitted due to mechanical damage (Rodriguez-Saona et al. 2001; El-Wakeil et al. 2003).
The activity of Trichogramma species was examined on different cotton cultivars which had various morphological and chemical traits were treated by jasmonic acid. The main objectives of the present study were
-
To test the influence of cotton plant physical characteristics (leaf position, distance and position of host egg, gland and trichome densities) on Trichogramma host searching efficiency.
-
To study the effect of cotton plant phytosterol composition on parasitization efficiency of Trichogramma species.
-
To investigate whether larval damage or jasmonate applications influence the plant volatile profile and alter the behaviour of Trichogramma species on different cotton cultivars.
Material and methods
Cotton plants
Gossypium barbadense (L.) of different cultivars (Giza 89, Giza 86 and Alex 4) which vary in gland and hair density on leaves (Table 1) were grown in 13 cm diameter pots containing a mixture of sand and clay (60:40% volume) in greenhouse (27–30°C & 14:10 L.D). Four to six weeks after sowing, plants with four to six fully expanded leaves were used for the experiments outlined hereafter.
Heliocoverpa armigera rearing
Adult Helicoverpa armigera (Hübner) were maintained on a sugar solution under laboratory conditions for the production of eggs. After hatching, the larvae were reared in square boxes 15 mm ´ 15 mm on an artificial diet according to Shorey and Hala (1965). These boxes were kept in a chamber regulated at 27 ± 2°C, 70% RH and 14:10 L:D.
Rearing of Trichogramma species
Trichogramma pretiosum (Riley) and Trichogramma minutum (Riley) were reared on Sitotroga cerealella (Olivier) eggs, attached to the surface of sticky paper strips (glue Tragant,Fluka, Germany) inserted into 50 ml glass vials 24 h after adult parasitoid emergence. The glass vials were closed and incubated at 27 ± 2°C, 70% RH and 14:10 L:D photoperiod for 8–10 days depending on life cycle duration of Trichogramma species. Female parasitoids, which were newly hatched from Sitotroga eggs, were used in the following experiments.
Effects of vertical host egg placement on Trichogramma efficiency
To test for parasitism site preference, vertical host egg placement on cotton plants was manipulated. Ten Helicoverpa eggs were fixed using adhesive material to the edge of each of the six fully expanded leaves of each cotton plant (six plants) of two cultivars (Giza 86 and Giza 89). Plants were then caged (60 ´ 100 ´ 70 cm) and directly exposed to the respective Trichogramma species at the ratio of 2:1 (female parasitoids: host eggs) for parasitism. After four to five days, the number of dark coloured Helicoverpa eggs was counted per leaf and the parasitism rate calculated in relation to the number of deposited eggs.
Effects of horizontal host egg placement on Trichogramma efficiency
In the second experiment, the distance between the placement of host eggs (on the lower surface of cotton leaves) and the release site for parasitoids (under the first row (0 m)) was manipulated. Three cotton cultivars (Giza 89, Giza 86 and Alex 4) were arranged each in three rows at 3.5 m intervals. Six plants were used in each row as replication for three cultivars. Parasitoids of both species were released at either a 2:1 or 5:1 ratio (female parasitoids:host eggs) at the first row to test for horizontal variation in parasitism. After 4–5 days, the number of dark coloured Helicoverpa eggs was counted per plant and the parasitism rate calculated in relation to the number of deposited eggs.
Effects of cotton plant gland and trichome density on parasitism rate of Helicoverpa eggs by Trichogramma
Ten Helicoverpa eggs were attached as described above on the 1st, 3rd and 5th leaves (from base to terminal) of Giza 89, Giza 86 and Alex 4 and exposed to the respective Trichogramma species, at the ratio of 2:1 female to host eggs for parasitization. Six plants were used as replicates of each cultivar. After four to five days, black eggs were counted to calculate the parasitism rates per leaf. The number of black glands and trichomes within a circle of 1 cm diameter at five sites on each leaf were counted to estimate their respective densities and the data were subjected to simple correlation analysis with the parasitism rates.
While it seems varying in gland and trichome densities of different cotton cultivars, as shown in table 1. It was very interesting to know whether gland and trichome densities affect Trichogramma efficiency. Therefore, an experiment was conducted with selected glandless cotton plants for the cultivar Alex 4 to compare them with the glanded plants of the same cultivar. The differences in trichome and black glands density may persuade Trichogramma to parasitize the host eggs. Six replicates were used in this experiment, both Trichogramma species were released on the attached Helicoverpa eggs, and then parasitism rate was calculated.
Effects of jasmonate application and larval damage on parasitization efficiency of Trichogramma species
The cultivars used in this experiment were Giza 86, Giza 89. Plant defenses were either induced by Helicoverpa larvae feeding or by treating with Methyl jasmonate.
Herbivore-damaged plants
A cohort of recently moulted fourth instar H. armigera from the colony was transferred from artificial diet cups to cotton plants (2 larvae/plant) 4 h prior to the experiment to allow the larvae to habituate to the new diet, and then placed inside a collection chamber containing a plant and allowed to feed for 24 h; ten plants were used as replicates.
Methyl jasmonate-treated plants
Each plant was treated by applying 20 µl of acetone–MeJA (8:1) (Sigma-Aldrich, Germany) overnight (Thaler et al. 1996) onto a 15 cm cotton tipped applicator in the greenhouse. Ten plants of each cultivar were used. Control cotton plants were exposed to 40 µl acetone. Ten host eggs were then mounted on each treated leaf as described above and exposed to the respective Trichogramma species (at ratio 1:1 host egg:parasitoid female) for parasitism rate.
Analysis of free phytosterols and volatiles
Free phytosterols were extracted from leaves (1st, 3rd and 5th ) of cotton cultivars (Giza 86, Giza 89 and Alex 4) and then quantified by the procedure described in Dugassa-Gobena et al. (1996) applying GC (Shmadzu 14A) equipped with FID, a capillary column (SPB1) and auto sampler. Solution of 100 µg/l d7-cholesterol in methanol was used as an internal standard. Six plants were used as replicates in each cotton cultivar. Phytosterols were identified by comparing the mass spectrometry obtained from the measurement by GC/MS at the Institute of Organic Chemistry (Göttingen University) with the mass spectrometry from the online database library for sterols according to National Institute of Standards and Technology (NIST 1995).
Plant volatiles were collected from an individual plant by closed loop stripping method as described by Boland et al. (1984) using a charcoal trapping tube. Volatiles were eluted from the charcoal with 150 µl of Dichloride methane and analysed with a GC. Camphor was used as an internal standard (152.4 µg of in 5 µl of Dichloride methane).
The instrument conditions were: Shimadzu model GC-14A; column: (SBP1 30 m ´ 0.32 mm ID, 0.25 µm film); column temperature program: 60°C for 3 min, then ramps to 280°C at 6°C/min and maintained for 10 min.; carrier gas was He at a linear flow velocity of 40 ml/min.
Furthermore, selected samples were analyzed by TRACE-GC/MS from Thermo Finnegan at the Institute for Organic Chemistry. GC/MS was equipped with the electron impact ionisation mass selective detector and DB5-MS capillary column (25 m ´ 0.25 mm ID, with a 0.25-µm-thick film). The temperature program was run with an initial temperature of 60°C for 3 min, then ramps to 280°C at 6°C/min, and maintained for 10 min. The spectral data of phytosterols and plant volatiles were used for the identification of respective compounds by comparing with commercially available standards spectrum from the database at the National Institute of Standards and Technology (NIST 1995) database.
Statistical analyses
Means were compared using a split plot design followed by Bonferroni correction, using the program SYSTAT 11 (Wilkinson et al. 2006). Percentage data were arcsine transformed prior to analyses. Correlation between parasitization rates and gland and trichome densities was also conducted by SYSTAT 11. Significant differences were noted at P < 0.05 for all trials.
Results
Vertical host eggs location and parasitizing efficiency of Trichogramma species
Manipulating the vertical placement of Helicoverpa eggs on the cotton plants had an influence on parasitization rates by Trichogramma species. Trichogramma were more likely to parasize Helicoverpa eggs (df = 23, P < 0.0354) on the lower and upper leaves than on the leaves in the middle position of cotton plants (Fig. 1). T. minutum had lower parasitization efficiency than T. pretiosum, which was further influenced by cotton cultivar. Both Trichogramma species showed higher (df = 3, P < 0.0059) parasitization efficiency on Giza 89 than Giza 86 cultivar.
Horizontal host eggs location and parasitism efficiency of Trichogramma species
There was a negative correlation (r = −0.72, P < 0.032) between the parasitization efficiency and the distance covered during location of host eggs by the parasitoids (Fig. 2). Host eggs parasitized at the highest rate were at the realign site, i.e. on plants at the first row for both Trichogramma species. The number of females released also influenced the parasitization efficiency (df = 17, P < 0.0362) of both species over the effect of host location. The larger the number of females released, the higher the number of Helicoverpa eggs parasitized. T. pretiosum parasitized more H. armigera eggs than T. minutum (df = 17, P < 0.0489). Parasitization rates for both parasitoids were influenced by location of Helicoverpa eggs on cotton cultivars (df = 35, P < 0.0023). Parasitism rates were the highest on Alex 4 cultivar, followed by Giza 89 and lastly Giza 86 was the lowest for both Trichogramma species on H. armigera eggs (Fig. 2).
Cultivars variation in trichome and blank gland densities of leaves
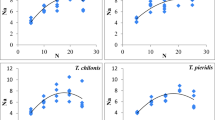
Figure 3 presents the trichome density for cotton cultivars on three physiologically aged leaves lower (1st), middle (3rd) and upper (5th). The result shows that for all cultivars, the trichome density per defined leaf area increases from the lower to the upper leaves. Nevertheless, cotton cultivars significantly differ (df = 8, P < 0.0414) from each other in their trichome density for the respective leaf position. Thus the leaves of Giza 86 had a greater density of trichome than the leaves of Alex 4 and Giza 89 (Table 1).
Figure 4 shows the number of black glands for three cotton cultivars and respective leaf position. The number of black glands increases from the lower leaves to the upper leaves for all cotton cultivars (df = 8, P < 0.0393). Leaves of Alex 4 and Giza 89 hold more black glands than leaves of Giza 86 (Table 1).
Results presented in Tables 2 and 3 reveal that the parasitization rate was low on cultivar Giza 86, with high trichome density and few black glands compared to cultivars Alex 4 and Giza 89 which had lower trichome density and more black glands (df = 17, P < 0.0427). Nevertheless, there was no difference between Trichogramma species. Thus trichome and black gland densities on leaves of cotton cultivars strongly influence the efficiency of Trichogramma species to parasitize Helicoverpa eggs.
Both Trichogramma species parasitize host eggs at a higher rate on glanded than glandless plants (df = 3, P < 0.0439) (Fig. 5). Yet there was a significant difference (df = 3, P < 0.0447) between Trichogramma species in parasitization efficiency, with T. pretiosum being more efficient than T. minutum.
Phytosterol components of cotton leaf
The results revealed that all cotton cultivars possess the same Phytosterol profile with β-sitosterol being the major component. Hexamethyl, β-Methyl-Cholesterol, Stigmasterol, β-Amyrin and Viminalol were also detected in less quantity compared to β-sitosterol. Linolenic acid, which was co-detected with phytosterols, was present in leaf extracts in a higher concentration than all respective sterol (Fig. 6).
Although the cotton cultivars possess a similar phytosterol profile, their contents were significantly different (df = 62, P < 0.0391) as well as for various leaf positions. Thus the cultivar Giza 86 retains for all leaf positions the lowest content of all phytosterols lower than Giza 89 and Alex 4, except for β-Amyrin and β-Methyl-Cholesterol. β-Amyrin was detected at a high concentration for Giza 86, especially in the younger 5th leaf as compared to both Giza 89 and Alex 4. Independent of the cotton cultivars, the older leaves (1st) showed significantly lower (df = 20, P < 0.0394) content of phytosterols than younger (5th) leaves. The high content of phytosterols in the younger leaves was primarily due to relatively high levels of β-sitosterol, β-Amyrin and Viminalol.
The cotton cultivars showed significant difference (df = 8, P < 0.0347) in the content of linolenic acid. Compared to the other phytosterol components, the lowest concentration of linolenic acid was also detected in Giza 86. Therefore, the phytosterol composition of cotton cultivars is investigated in these experiments in an attempt to explain the variation of parasitization efficiency of Trichogramma on Helicoverpa eggs (Table 2).
Volatiles induced by larval damage and methyl jasmonate (MeJA) treatments
Differences in volatile emissions were observed for cotton plants damaged by H. armigera larvae, treated with MeJA, or undamaged controls. Control plants emitted much lower volatiles compared to herbivore-damaged plants or MeJA treated plants. There was also a great variation in volatiles emitted by larvae damaged and MeJA treated plants (df = 5, P < 0.0379). Plants treated with herbivores or MeJA emitted a blend that consisted of Hexanal-2ethyl; β Myrcene, Cis-3-hexenyl acetate, β-ocimene, linalool, (E)-4,8-dimethyl-1,3,7-nonatriene, Methyl salicylate, Indole, (E)-β-Caryophyllene, Trans-β-farnesene, and σ-Cadinene (Fig. 7). Hexanal, (Z)-3-hexenol, and hexyl acetate, all Lipoxygenase pathway compounds, were detected in response to herbivore damage, but showed a small response to MeJA treatment (Fig. 7).
Volatiles collected from undamaged cotton plants (control), plants damaged by H. armigera and plants treated with Jasmonate. Volatiles were collected for 24 h and identified based on NIST. Each bar represents mean ± SE 1: Hexanal-2ethyl; 2: ß Myrcene; 3: Cis-3-Hexenyl acetate; 4: ß Ocimene; 5: Linalool; 6: (E)4,8 Dimethyl-1,3,7 Nonatriene;7: methyl salicylate; 8: Indole; 9: (E)ß Caryophyllene; 10: Trans-ß-farnesene and 11: Cadinene a Giza 89 and b Giza 86. Different letters indicate significant differences (P < 0.05)
There was a relationship between emitted volatiles and parasitism rates of Trichogramma species on Helicoverpa eggs on cotton cultivars Giza 89 and Giza 86. Generally, the induced volatiles were higher (df = 5, P < 0.0321) in Giza 89 than Giza 86 plants; therefore, parasitism rates were also higher (df = 5, P < 0.0211) on Giza 89 than Giza 86, as shown in Table 4.
Discussion
Parasitism rates of Trichogramma on cotton leaves were the highest on the 1st, 2nd, 5th and 6th leaves, while they were lowest on the middle leaves (3rd and 4th). This result corresponds to that of Ables et al. (1980) and Gingras et al. (2008), who reported that the broadly comparable structure of different leaf positions may influence plant–herbivore–parasitoid interactions. T. pretiosum was more efficient than T. minutum in finding hosts. Cotton cultivars could be arranged for their attractiveness of Trichogramma as follows: Alex 4, Giza 89 and Giza 86.
The level of parasitism was negatively correlated with distance that the sentinel eggs were located from the release point. There are several possible explanations for this. Firstly, the parasitoids may have taken more time to find eggs further away from release point. Secondly, once wasps encountered nearby eggs, they may have spent time handling their hosts before continuing to forage as has been proposed by Andow and Prokrym (1990) for T. nubilale. The decrease of parasitism with increasing distance in this experiment implies that for inundative releases, the distance between release points must be taken into consideration to maximize parasitism rates of Trichogramma species in biological control programs.
Trichogramma behaviour was affected by leaf cotton trichome and black gland densities. Parasitism rates correlated negatively with leaf cotton trichome, and positively with black gland density. These results are consistent with Romeis and Shanower (1996) and Romeis et al. (1998), who stated that trichome density, may inhibit parasitoid searching behaviour. Based on the present results, leaf trichome density may reduce Trichogramma host location ability for Helicoverpa eggs, and black glands may attract Trichogramma to parasitize Helicoverpa eggs. These results correspond with those of Mohite and Uthamasamy (1998), who mentioned that rates of parasitism were negatively associated with trichome density, when they studied the interaction between eight wild species of Gossypium and a cultivated cotton cultivar MCU 9, resistant to H. armigera, and the pest’s natural enemies. Theses results are also similar to Romeis et al. (1996), who demonstrated that trichome numbers were responsible for low parasitism rates of Trichogramma on H. armigera on pigeonpea plants. Leaf morphological traits are one option for selection by plant breeders to produce cultivars, with a high density of glands and low density of trichomes; this would increase Trichogramma parasitism to control Helicoverpa into cotton.
Parasitism rates were higher on glanded than glandless plants. Plant surfaces contain epicuticular waxes, chemicals produced internally and leached into the leaf wax, and compounds actively exuded to the plant surface including trichome exudates as recorded by Eigenbrode and Espelie (1995). Attractiveness of Trichogramma to glandless and gland plants is unlikely. This may be due to the glandless-plant structure negatively affecting Trichogramma host location behaviour while glanded plants may have special chemical volatiles to attract more wasps.
Trichogramma females do not reject the host while examining, types of plant hosts; glanded and glandless are accepted, but the difference between two plant types appears in parasitism rates of Trichogramma. This result was consistent with that found by Romeis et al. (1996), who mentioned that leaves mainly possess short and erect non-glandular trichomes which are more densely spaced on the lower than on the upper surface. Another opinion is that by Trichogramma species accept or reject host eggs is during the examining phase. Criteria that deter attack are probably assessed at this time. Females were never observed to reject hosts once oviposition had commenced; Pak and de Jong (1987) noted the same phenomenon. The parasitoids prefer to walk on the leaf margins and on the major veins of the lower leaf surface. In contrast to the interveinal areas, veins and margins are covered with long non-glandular trichomes which are appressed to the surface as confirmed by Romeis et al. (1996), making walking easier on these structures.
Significant differences in phytosterols compositions were found between cotton cultivars and leaf positions. There was a relationship between phytosterol components and parasitism rates. Parasitism rates were positively related to Linolenic acid, Hexamethyl, Stigmasetrols, β-Sitosterols and Viminalol, and negatively related to β-Methyl Cholesterols and β-Amyrin. These results correspond to those of Karban and Baldwin (1997) and Schmelz et al. (2009), who reported that most phytophagous insects and their natural enemies make some sensory exploration of the leaf surface before sitting on the wax layer of leaf surfaces. Waxes also produce compounds which attract the parasitoids or predators of phytophagous insects.
In fact, a blend of volatiles released by cotton plants treated exogenously with jasmonate contained all compounds released during herbivore damage. This result is consistent with Thaler (1999) and Rodriguez-Saona et al. (2001); who reported that volatiles emitted from jasmonate application are similar to those released during larval damage, and serve as essential host-location cues for parasitic insects. Contrary to this Methyl jasmonate did not induce emissions of stored terpenes, a result that was expected since the release of stored terpenes is dependent on physical damage caused by herbivores as mentioned by Paré and Tumlinson (1997). It is therefore likely that the entire blend of odours released by a specific plant can be used as a signal. Herbivore-infested plants selectively attract parasitoids as confirmed by De Moraes et al. (1998) and Kessler and Baldwin (2001). Parasitoids of herbivores could use these volatiles as cues to pinpoint the location where the herbivores were last feeding.
In conclusion, parasitism rates were negatively correlated to the distance between the releasing site of Trichogramma and egg batches on host plants. Morphological traits, i.e. presence of black glands or trichome densities played a significant role. Jasmonate can activate indirect defences in cotton. Volatiles released from cotton after herbivore feeding and induced by jasmonate treatment provide cues for attracting natural enemies. For instance, in other plant systems, indirect evidence has been presented that volatiles induced by jasmonate serve as important cues for Trichogramma wasps to locate potential host sites. Thus, it appears that jasmonate are potential agents that may be used to improve biocontrol in cotton production.
References
Ables JR, McCommas DW, Jones SL, Morrison RK (1980) Effect of cotton plant size, host egg location and location of parasite release on parasitism by Trichogramma pretiosum. Southwest Entomol 5:261–264
Andow DA, Prokrym DR (1990) Plant structural complexity and host-finding by a parasitoid. Oecologia 82:162–165
Bilger W, Veit M, Schreiber L, Schreiber U (1997) Measurement of leaf epidermal transmittance of UV radiation by chlorophyll fluorescence. Physiol Plant 101:754–763
Boland W, Ney P, Jaenike L, Gassmann L (1984) A “closed-loop-stripping” technique as a versatile tool for metabolic studies of volatiles. In: Schreier P (ed) Analysis of volatiles. Walter de Gruyer, Berlin, pp 371–380
Creelman RA, Mullet JE (1997) Biosynthesis and action of jasmonates in plants. Ann Rev Plant Physiol Plant Mole Biol 48:355–381
De Moraes CM, Lewis WJ, Pare PW et al (1998) Herbivore-infested plants selectively attract parasitoids. Nature 393:570–573
Dicke M (1999) Are herbivore-induced plant volatiles reliable indicators of herbivore identity to foraging carnivorous arthropods? Entomol Exp Appl 91:131–142
Dugassa-Gobena D, von Alten H, Schönbeck F (1996) Effects of arbuscular mycorrhiza (AM) on health of Linium usitatissimum. Plant & Soil 185:173–182
Eigenbrode SD, Espelie KE (1995) Effects of plant epicuticular lipids on insect herbivores. Ann Rev Entomol 40:171–194
El-Wakeil NE, Bernal J, Vidal S (2003) Effects of jasmonate applications on pest and natural enemy recruitment in cotton fields. Proc of the World Cotton Res Con-3, from 9 to 13 March 2003. Cape Town, South Africa, pp 1239–1248
Gingras D, Boivin G (2002) Effect of plant structure, host density and foraging duration on host finding by Trichogramma evanescens. Environ Entomol 31:1153–1157
Gingras D, Dutilleul P, Boivin G (2002) Modeling the impact of plant structure on host-finding behaviour of parasitoids. Oecologia 130:396–402
Gingras D, Dutilleul P, Boivin G (2003) Effect of plant structure on host finding capacity of lepidopterous pests of crucifers by two Trichogramma parasitoids. Bio Control 27:25–31
Gingras D, Dutilleul P, Boivin G (2008) Effect of plant structure on searching strategy and searching efficiency of Trichogramsma turkestanica. J Insect Sci 8:1–9
Grieshop MJ, Flinn PW, Nechols JR, Schöller M (2007) Host-foraging success of three species of Trichogramma in a simulated retail environment. J Econ Entomol 100:591–598
Karban R, Baldwin I (1997) Induced responses to herbivory. The University of Chicago Press, Chicago, p 319
Kessler A, Baldwin IT (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291:2141–2144
Lukianchuk JL, Smith SM (1997) Influence of plant structural complexity on the foraging success of Trichogramma minutum a comparison of search on artificial and foliage models. Entomol Exp Appl 84:221–228
Mohite PB, Uthamasamy S (1998) Host-plant resistance and natural enemies interaction in the management of Helicoverpa armigera on cotton. Indian J Agric Res 32:28–30
Mustaparta H (1984) Olfaction. In Bell WJ, Cardé RT (eds) Chemical ecology of insects. Chapman & Hall, London, pp 483–520
NIST (National Institute of Standards and Technology) (1995) Mass spectral library on CD-rom, version 1.0. NIST, Gaithersburg, Maryland
Pak GA, de Jong EJ (1987) Behavioural variation among strains of Trichogramma spp.: host recognition. Neth J Zool 37:137–166
Paré PW, Tumlinson JH (1997) Induced synthesis of plant volatiles. Nature 385:30–31
Paré PW, Tumlinson JH (1998) Cotton volatiles synthesized and released distal to the site of insect damage. Phytochemistry 47:521–526
Paré PW, Tumlinson JH (1999) Plant volatiles as a defence against insect herbivores. Plant Physiol 121:325–331
Rodriguez-Saona C, Crafts-Brandner SJ, Paré PW, Henneberry TJ (2001) Exogenous methyl jasmonate induces volatiles emissions in cotton plants. J Chem Ecol 27:679–695
Romeis J, Shanower TG (1996) Arthropod natural enemies of Helicoverpa armigera in India. Biocontrol Sci Technol 6:481–508
Romeis J, Shanower TG, Peter AJ (1996) Type and distribution of trichomes on pigeonpea leaves. Intern Chickpea Pigeonpea Newslett 3:101–102
Romeis J, Shanower TG, Zebitz CPW (1997) Volatile plant infochemicals mediate plant preference of Trichogramma chilonis. J Chem Ecol 23:2455–2465
Romeis J, Shanower TG, Zebitz CPW (1998) Physical and chemical plant characters inhibiting the searching behaviour of Trichogramma chilonis. Entomol Exp Appl 87:275–284
Romeis J, Babendreier D, Waeckers FL, Shanower TG (2005) Habitat and plant specificity of Trichogramma egg parasitoids underlying mechanisms and implications. Basic Appl Ecol 6:215–236
Runyon JB, Mescher MC, De Moraes CM (2008) Parasitism by Cuscuta pentagona attenuates host plant defenses against insect herbivores. Plant Physiol 146:987–995
Schmelz EA, Engelberth J, Alborn HT, Tumlinson JH, Teal PEA (2009) Phytohormone-based activity mapping of insect herbivore-produced elicitors. PNAS 106:653–657
Shorey HH, Hala RL (1965) Mass rearing of some noctuid species on a simple artificial medium. J Econ Entomol 58:522–544
Smith SM (1996) Biological control with Trichogramma: advance, successes, and potential of their use. Ann Rev Entomol 41:375–406
Takabayashi J, Dicke M, Posthumus MA (1994) Volatile herbivore-induced terpenoids in plant–mite interactions: variation caused by biotic and abiotic factors. J Chem Ecol 20:1329–1354
Thaler JS, Stout MJ, Karban R, Duffey SS (1996) Exogenous jasmonates simulate insect wounding in tomato plants in the laboratory and field. J Chem Ecol 22:1767–1781
Thaler JS (1999) Jasmonate-inducible plant defences cause increased parasitism of herbivores. Nature 399:686–688
Thorpe KW (1985) Effects of height and habitat type on egg parasitism by Trichogramma minutum and T. pretiosum. Agric Ecosys Environ 12:117–126
Turlings TCJ, Loughrin JH, Mccall PJ, Roese USR, Lewis WJ, Tumlinson JH (1995) How caterpillar-damaged plants protect themselves by attracting parasitic wasps. PNAS 92:4169–4174
Vet LEM, Dicke M (1992) Ecology of infochemical use by natural enemies in a tritrophic context. Ann Rev Entomol 37:141–172
Vet LEM, de Jong AG, Franchi E, Papaj DR (1998) The effect of complete versus incomplete information on odour discrimination in a parasitic wasp. Animal Behav 55:1271–1279
Wilkinson L, Hill MA, Vang E (2006) SYSTAT: STATISTICS; version 11.0 edition. Evanston (IL), SYSTAT Software Inc
Acknowledgments
I would like to thank Prof. S. Vidal (Plant Pathology and Protection Institute, Göttingen University) for providing me place and facilities to conduct this research in his institute and for useful comments on this MS. Thanks also to Dr. Frauendorf, Organic Chemistry Institute (Göttingen University) for his assistance and advices in GC MS analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
El-Wakeil, N. Impacts of cotton traits on the parasitization of Heliocoverpa armigera eggs by Trichogramma species. Gesunde Pflanzen 63, 83–93 (2011). https://doi.org/10.1007/s10343-011-0250-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10343-011-0250-1
Keywords
- Cotton
- Helicoverpa armigera
- Searching behaviour
- Trichogramma pretiosum
- T. minutum
- Volatile and sterols compounds