Abstract
Sweetpotato weevil (SPW) pest management is challenging because the pest target is sub-terranean, so the application of pesticides is impractical and usually ineffective. Host plant resistance and the genetic transformation of sweetpotatoes to produce entomotoxic Bt proteins offer potential for environmentally benign pest control. Resistance can be conferred by naturally occurring hydroxycinnamic acids which protect against oviposition by adults, but these compounds are restricted to the root surface so do not protect against the cortex bound larvae where the greatest damage occurs. Resistance could be enhanced if combined with expression of Bt proteins in transformed plants, but interactions between hydroxycinnamic acids and Bt proteins remain unknown. Here the bioactivity of Cry7Aa1 protein and hydroxycinnamic acid esters was evaluated individually and in combination against SPW larvae and mortality determined. Low and high concentrations of hydroxycinnamic acid esters alone caused significantly higher mortality of both weevil species in all experiments compared to the control. SPW larval mortality was greater when tested as a combination of hydroxycinnamic acid esters and Bt protein, but this effect was additive not synergistic. Although we report no evidence of antagonistic interactions, the antifeedant effects of the plant compounds conferring host plant resistance could have reduced consumption of the Bt protein in our assays leading to a lower efficacy when combined. Further work is required to determine whether the toxic effects of Bt proteins function alongside host plant resistance in sweetpotato under field conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
-
Host plant resistance and GM crops provide alternatives to synthetic pesticides for controlling pests of sweetpotato, but their interactions in combination are unstudied.
-
Hydroxycinnamic acid esters were shown to mediate resistance, but cortex concentrations were too low to protect against damage by larvae.
-
Bt protein was bioactive against sweetpotato weevil larvae so could compliment the effects of hydroxycinnamic acids.
-
Bt protein and hydroxycinnamic acids combined in an additive rather than synergistic way, and no significant antagonistic interaction was recorded.
Introduction
Sweetpotato (Ipomoea batatas) (L.), one of the world’s most important food crops, ranked seventh globally (Loebenstein 2009). Sweetpotato is high yielding with wide adaptation to marginal soils and is highly resistant to drought (Placide et al. 2013; Laurie et al. 2015). It is also an important staple in many countries underpinning food and nutritional security and is a notable source of vitamins C, B2 (riboflavin), B6, beta-carotene (the vitamin A precursor abundant in orange fleshed varieties), as well as dietary fibre, potassium, copper, manganese and iron (Loebenstein 2009).
While production of sweetpotato is limited by a range of pests and diseases, the major constraints are sweetpotato weevils (SPW), Cylas spp. (Fite et al. 2014; Kiiza et al. 2009; Nottingham and Kays 2002). This insect damages vines, storage roots and occasionally the foliage, reducing both yield and quality of the crop and causing potentially total yield losses in East Africa (Stathers et al. 2003a, 2003b; Rees et al. 2003 and Smit 1997). The primary damage is reportedly caused by SPW larvae tunnelling inside the root cortex which triggers the production of distasteful sesquiterpenes by the sweetpotato root (Sato and Uritani 1981; Uritani et al. 1975). This is the source of the bitter taste that makes the infested root unfit for both human and livestock consumption (Pandey 2009; Woolfe 1992), but there is no evidence that these compounds affect infestation by SPW.
SPW control using conventional pesticides is complicated because sweetpotato roots are subterranean, and the target insect spends the majority of its lifecycle inside roots protected by the soil. Hydroxycinnamic acids esters (hexadecyl and octadecyl-cinnamic acid esters, hexadecylcaffeic acid esters and 5-O-caffeoylquinic acid) are produced naturally by the roots and are biologically active against SPW larvae at ecologically relevant concentrations (Stevenson et al. 2009) and have been identified as a breeding trait for improved varieties (Otema et al. 2017; Yada et al. 2017). These compounds are particularly effective at protecting against oviposition by adults because they occur at the highest concentrations at the root surface where eggs are laid and induce behavioural avoidance after feeding and creating feeding holes in which eggs are laid (Anyanga et al. 2013) but are less effective against the larval stage which causes the most damage in the cortex. Host plant resistance could in this case be complimented by the use of biologically active Bacillus thuringiensis (Bt) proteins expressed in the cortical tissue through transformation of resistant varieties giving the roots protection against both adults at the surface and tunnelling larvae in the cortex. Combining nature-based insect control measures can enhance their efficacy as reported recently for pyrethrins and the entomopathogenic fungus Metarhizium anisopliae (Fernandez-Grandon et al. 2020). Additionally, host plant resistance could reduce the likelihood of pests developing resistance to Bt proteins that otherwise can happen relatively rapidly under certain conditions (Bravo et al. 2007). Several crops have already been transformed to express Bt Cry proteins, including crops that express coleopteran-active Cry proteins in roots against Diabrotica spp. in maize, Zea mays L. (Cry3Bb1, mCry3A, and Cry34/35Ab1), and sweetpotato weevil, C. formicarius, in sweetpotato (Cry3A) (EPA 2018; Moran et al. 1998; Vaughn et al. 2005; Storer et al. 2006). Sweetpotato expressing Cry3A was not developed further, partly because the Cry3A protein expressed within the sweetpotato root results in relatively low C. formicarius control (Moran et al. 1998). However, Ekobu et al. (2010) reported that Cry7Aa1, ET33/34, and Cry3Ca1 had LC50 values of 1 μg/g added in diet against larvae of C. puncticollis indicating potential for these proteins in sweetpotato. Cry7Aa1 has been the candidate of transformation trials in sweetpotato and so presents the most promising entomotoxic Bt protein to combine with host plant resistance (Rukarwa et al. 2013).
Interactions between plant defence compounds and biopesticides are reported with potentially detrimental effects (Stevenson et al. 2010). Interactions between Cry7a proteins produced by Bt transformed sweetpotato varieties and the hydroxycinnamic acid defence compounds could also occur with potential antagonistic or synergistic effects if used together in roots, but this remains an important knowledge gap. Therefore, the objective of this study was to evaluate the interaction of hydroxycinnamic acids with Cry7a proteins and determine their effects on weevil larval mortality. We also determined the effects of the plant compounds against larvae and SPW adult feeding and oviposition which has previously not been reported.
Materials and methods
Evaluation of effects of hydroxycinnamic acid esters on adult SPW feeding and oviposition
Roots of NASPOT1, a SPW susceptible sweetpotato variety, were used for the experiment for testing the hydroxycinnamic acid esters because they are fed upon by SPW and provide a food medium for testing plant compounds but are naturally low in these chemicals (Stevenson et al. 2009; Anyanga et al. 2013). Root cores were obtained by cutting sweetpotato roots using a 24-mm-diameter cork-borer No.15 (Stevenson et al. 2009). The root core was used for testing effects of hydroxycinnamic acid esters on adult SPW feeding and oviposition.
Twelve root cores of the susceptible variety NASPOT1 placed in a 12-well tray were treated with three different concentrations of octadecylcaffeic and coumaric acid, heptadecylcaffeic acid and 5-O-caffeoylquinic acid synthesized at the Natural Resources Institute (UK) as reported previously (Stevenson et al. 2009). Individual hydroxycinnamic acid esters were weighed and 1.0 mg dissolved in 1.0 mL of acetone as a carrier solvent to obtain a concentration of 1 mg/ml that was serially diluted following Anyanga et al. (2013). The experiment was set up in a completely randomized design. Each experimental set up was replicated 15 times. Twenty-four adult (2-weeks-old) gravid female SPW were introduced into a 12-well feeding trays representing 2 SPW per root core and allowed to feed and oviposit for 24 h and 48 h for C. puncticollis and C. brunneus,, respectively, owing to the pest size and thus rate at which the target pests damage the root core (Anyanga et al. 2013; Nottingham et al. 1987).
Evaluation of interactive effects of hydroxycinnamic acid esters and Cry7Aa1 Bt proteins on SPW larval mortality or survival
Sweetpotato flour was obtained by grinding dry chips of NASPOT1 a susceptible variety following Ekobu et al. (2010). Three hydroxycinnamic acid esters: hexadecylcaffeic acid, hexadecylcoumaric acid and octadecylcoumaric acid were synthesized after Stevenson et al. (2009), while 5-O-caffeoylquinic acid was obtained from Sigma-Aldrich (Dorset, UK). Diet for this experiment was prepared following procedures reported by Ekobu et al. (2010). Agar was heated to 100 °C and cooled to 55 °C before mixing in other ingredients including sweetpotato flour (180 g), casein (24 g), cellulose (16 g), sucrose (40 g), yeast (10 g), salt mixture (3.0 g) and ascorbic acid (2.0 g) using a magnetic stirrer.
Additional ingredients: B-vitamin mixture (40 mg), choline chloride (400 mg), inositol (320 mg), cholesterol/stigmasterol (640 mg), potassium sorbate (600 mg), tetracycline (200 mg) and methylhydroxybenzoate (675 mg), were mixed in 20 mls of 100% ethanol by stirring on non-heat shaking equipment at National Crops Resources Research Institute (NaCRRI) sweetpotato tissue culture laboratory. The ethanolic mixture was added to agar mixture and blended for 10 min. Ninety grams (90 g) of the molten diet was poured in to petri dish and allowed to solidify.
A stock solution of test hydroxycinnamic acids was prepared by weighing 10 mg of the compound and dissolving in 10 ml of acetone to make 1 mg ml−1 of solution. The solution was serially diluted to make 0.1 and 0.01 mg ml−1 solution for the experiment. Bt-protein stored in the refrigerator was prepared by diluting with water to obtain a 1 µg g−1 dilution as used by Ekobu et al. (2010). Nineteen treatment combinations were used to test for interactions in the experiment as follows:
-
Treatment 1. Diet only.
-
Treatment 2. Diet + 1 ml of acetone.
-
Treatment 3. Diet + 1 ml Bt-protein (1 µg g−1).
-
Treatment 4. Diet + 1 ml of 0.1 mg ml−1 C18 Coumaric acid esters.
-
Treatment 5. Diet + 1 ml of 0.01 mg ml−1 C18 Coumaric acid esters.
-
Treatment 6. Diet + 1 ml of 0.1 mg ml−1 C18 Coumaric acid esters + 1 µg g−1 Bt-protein.
-
Treatment 7. Diet + 1 ml of 0.01 mg ml−1 C18 Coumaric acid esters + 1 µg g−1 Bt-protein.
-
Treatment 8. Diet + 1 ml of 0.1 mg ml−1 C16 Coumaric acid esters.
-
Treatment 9. Diet + 1 ml of 0.01 mg ml−1 C16 Coumaric acid esters.
-
Treatment 10. Diet + 1 ml of 0.1 mg ml−1 C16 Coumaric acid esters + 1 µg g−1 Bt-protein.
-
Treatment 11. Diet + 1 ml of 0.01 mg ml−1 C16 Coumaric acid esters + 1 µg g−1 Bt-protein.
-
Treatment 12. Diet + 1 ml of 0.1 mg ml−1 C16 Caffeic acid esters.
-
Treatment 13. Diet + 1 ml of 0.01 mg ml−1 C16 Caffeic acid esters.
-
Treatment 14. Diet + 1 ml of 0.1 mg ml−1 C16 Caffeic acid esters + 1 µg g−1 Bt-protein.
-
Treatment 15. Diet + 1 ml of 0.01 mg ml−1 C16 Caffeic acid esters + 1 µg g−1 Bt-protein.
-
Treatment 16. Diet + 1 ml of 0.1 mg ml−1 Chlorogenic acid.
-
Treatment 17. Diet + 1 ml of 0.01 mg ml−1 Chlorogenic acid.
-
Treatment 18. Diet + 1 ml of 0.1 mg ml−1 Chlorogenic acid + 1 µg g−1 Bt-protein.
-
Treatment 19. Diet + 1 ml of 0.01 mg ml−1 Chlorogenic acid + 1 µg g−1 Bt-protein
Ten first instars of C. puncticollis (8 days) were obtained by allowing gravid female weevils to oviposit on sweetpotato roots (NASPOT 1) for 24 h. The age of the weevils was recorded from the first day of incubation. Ten small burrows were excavated using the spatula edge from the solidified agar media. One larva was placed into each diet with ten burrows per Petri dish. The displaced diet was replaced and inverted on top of the larva to avoid damage to the larva and minimize desiccation. The diets were then covered with a disc of filter paper to absorb excess moisture from the diets, and the lids were replaced on top. The bioassay was left to stand for 15 days and evaluated by observing larval movement following the procedure of Rukarwa et al. (2013). The experiment was conducted at 25 ± 2 °C and 70 ± 10% relative humidity in a completely randomized design replicated 3 times.
Data collection and analysis
Data were collected on the number of feeding holes and faecal droppings produced in 24 h and 48 h feeding by adult gravid female C. puncticollis and C. brunneus, respectively. The periderm of the root was gently removed, and the number of eggs laid was recorded using a magnifying glass. Data obtained on the number of feeding holes, faecal droppings and eggs laid were analysed using analysis of variance (ANOVA) linear model in R package. The statistical probability and the mean number of feed holes, faecal droppings and eggs laid were generated.
The number of dead larvae was counted as those that showed no motility and recorded as the percentage larval mortality from the proportion of the dead larvae compared to the total number of larvae and the results multiplied by 100. Means were generated, and mean separation test was done using least significant difference (LSD) at 5%.
To develop the model to test whether there was interaction, the data were analysed by looking at the presence or absence of Bt and different concentrations of hydroxycinnamic acid esters on larval mortality. The data were transformed, and a logistic regression model was used to analyse the data. Logistic regression models are Generalized Linear Models (GLM) with binomial random component and logit link;
Glm (formula = cbind (Pct-mortality) ~ Rep + Acid + Acid Concentration + Bt + Acid: Acid Concentrations + Acid: Bt + Acid Concentration: Bt + Acid: Acid Concentration: Bt, family = quasibinomial, data = Bt × 2).
Results
Effects of hydroxycinnamic acid esters on adult SPW feeding and oviposition
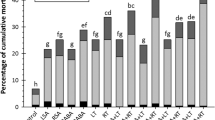
The number of C. puncticollis and C. brunneus feeding holes on root cores was significantly different (P < 0.05) between treatments. Overall, the mean number of C. puncticollis and C. brunneus feeding holes decreased with an increase in the concentration of octadecylcaffeic acid, with the least feeding holes on root cores treated with the highest concentration (0.1 mg ml−1) octadecylcaffeic acid (Fig. 1). Similarly, the mean number of C. puncticollis feeding holes was significantly higher (P ≤ 0.05) on control root surface than those treated with octadecylcoumaric acid with the mean number of C. puncticollis feeding holes decreasing as the concentration of octadecylcoumaric acid applied on the root surface increased, and it differed significantly between control and within the treatment levels (Fig. 1). The number of C. brunneus feeding holes on the root surface also differed significantly among treatments (P ≤ 0.001) showing a similar response to adults of C. puncticollis, being significantly higher (P ≤ 0.05) on the control root than on the roots treated with octadecylcoumaric acid (Fig. 1).
Mean number of feeding holes produced by C. puncticollis and C. brunneus on sweetpotato root plugs treated with different concentrations of octadecylcaffeic acid, octadecylcoumaric acid, heptadecylcaffeic acid and 5-O-Caffeoylquinic acid Cp: C. puncticollis, Cb: C. brunneus, Fhole: feeding hole, N = 15, ± SEM
There was a significant difference (P ≤ 0.001) in the number of feeding holes caused by both C. puncticollis and C. brunneus on the sweetpotato root core treated with heptadecylcaffeic acid (Fig. 1). The mean number of feeding holes caused by feeding of both weevil species decreased significantly with increasing levels of hexadecylcaffeic acid concentrations. The highest mean number of feeding holes was recorded on the control root plug (Fig. 1). The mean number of feeding holes caused by C. puncticollis and C. brunneus was similar at 0.1 mg ml−1 concentration of heptadecylcaffeic acid applied on the root core (Fig. 1). The mean number of C. puncticollis feeding holes differed significantly (P ≤ 0.001) between root cores treated with 5-O-caffeoylquinic acid and control. The mean number of feeding holes was significantly (P ≤ 0.05) higher on untreated root cores than on root cores treated with 5-O-caffeoylquinic acid (Fig. 1). The mean number of feeding holes in root exposed to C. brunneus feeding was higher compared to similarly treated roots where C. puncticollis were placed to feed on similarly treated root core suggesting that these octadecylcaffeic acid esters and 5-O-caffeoylquinic acid had a reduced effect on feeding behaviour of C. brunneus (Fig. 1). The mean number of feeding holes for both species was, however, similar on the root core treated with heptadecylcaffeic acid esters (Fig. 1).
Effects of hydroxycinnamic acids on production of faeces by Cylas puncticollis and C. brunneus
The number of faecal droppings was used as an additional indirect measure of the effect of natural plant compounds on insects feeding behaviour and development. The number of faecal droppings produced by C. puncticollis and C. brunneus was significantly lower on sweet potato root cores treated with octadecylcaffeic acid than on control root cores (P ≤ 0.001) (Fig. 2), and this effect was dose dependent. The mean number of C. puncticollis faecal droppings differed significantly (P ≤ 0.001) and in a similar way between octadecylcoumaric acid treated sweetpotato root surface and control. C. brunneus showed a similar pattern and was significantly different (P ≤ 0.001) (Fig. 2). Unlike the trend observed with other compounds, the number of C. puncticollis and C. brunneus faecal droppings produced on the surface of sweetpotato root core treated with heptadecylcaffeic acid was only significantly different (P ≤ 0.05) from the control treatment for C. brunneus at the highest concentration (0.1 mg ml−1). The number of C. puncticollis faecal droppings was lowest on the root core treated with 0.1 mg ml−1 heptadecylcaffeic acid (Fig. 2). The number of faecal droppings produced by either species decreased with increasing concentration of heptadecylcaffeic acid treatment (Fig. 2).
Mean number of faecal droppings produced on sweetpotato root cores treated with different concentrations of octadecylcaffeic acid, octadecylcoumaric acid, heptadecylcaffeic acid and 5-O-caffeoylquinic acid, FaecaldropsCp and FaecaldropsCb represents faecal droppings of Cylas puncticollis and C. brunneus, respectively
There was a significant difference (P ≤ 0.019) in the number of faecal droppings produced by C. puncticollis among treatments with 5-O-caffeoylquinic acid. The number of faecal droppings produced by C. brunneus was, however, not significantly different (P > 0.05) among the treatments. The mean number of faecal droppings was significantly (P ≤ 0.05) higher on the untreated root core than on the root core treated with 5-O-caffeoylquinic acid (Fig. 2). There were no faecal droppings produced by C. brunneus on the root core treated with 0.1 mg ml−1 5-O-caffeoylquinic acid.
Biological activity of hydroxycinnamic acids at different concentrations on oviposition by C. puncticollis and C. brunneus
C. puncticollis egg laying was significantly (P ≤ 0.023) different among the octadecylcaffeic acid treatments. Similarly, there was a significant difference (P ≤ 0.05) in oviposition of C. brunneus between treatments and the control. The mean number of C. puncticollis eggs laid on the untreated root core was significantly higher than on the root core treated with different concentrations of octadecylcaffeic acid. C. puncticollis did not oviposit any eggs at all on the root core treated with 0.01 and 0.1 mg ml−1 octadecylcaffeic acid, respectively (Fig. 3). The mean number of eggs laid by C. brunneus decreased significantly with increasing concentration of octadecylcaffeic acid (Fig. 3). The root core treated with 0.1 mg ml−1 of octadecylcaffeic acid prevented C. brunneus females from laying eggs completely (Fig. 3).
Similarly, octadecylcoumaric acid reduced C. puncticollis and C. brunneus oviposition compared to controls (P ≤ 0.001). The mean number of C. puncticollis eggs laid on the untreated root core was significantly (P ≤ 0.05) higher than on roots treated with octadecylcoumaric acid (Fig. 3). The mean number of eggs laid on the root core decreased with increasing concentration of octadecylcoumaric acid treatment on the periderm (Fig. 3). The lowest mean number of eggs was laid on the root core treated with 0.1 mg ml−1 octadecylcoumaric acid (Fig. 3).
There were significant differences (P ≤ 0.01) in the number of eggs laid by C. puncticollis on the roots treated with heptadecylcaffeic acid. The oviposition of C. brunneus was also significantly different (P ≤ 0.001) on the root cores treated with heptadecylcaffeic acid. The mean number of C. brunneus eggs laid on control root was significantly (P ≤ 0.05) higher than that recorded on root cores treated with heptadecylcaffeic acid (Fig. 3). C. puncticollis did not lay eggs at all on the root core treated with highest concentration 0.1 mg ml−1 of heptadecylcaffeic acid (Fig. 3). C. brunneus did not lay eggs on any root cores treated with different concentrations (0.001, 0.01 and 0.1 mg ml−1) of heptadecylcaffeic acid respectively (Fig. 3).
There was a significant difference (P ≤ 0.005) in oviposition of C. puncticollis and C. brunneus among treatments. The mean number of eggs laid by C. puncticollis was significantly higher (P ≤ 0.05) on the untreated root cores than on the root cores treated with 5-O-caffeoylquinic acid (Fig. 3). The mean number of eggs laid by C. brunneus followed a similar trend and was significantly (P ≤ 0.05) more on the untreated root cores than it was on the root cores treated with 5-O-caffeoylquinic acid (Fig. 3). Egg laying in both C. puncticollis and C. brunneus was completely inhibited at 0.01 and 0.1 mg ml−1 5-O-caffeoylquinic acid, respectively (Fig. 3).
Interactive effects of hydroxycinnamic acid esters and Cry 7Aa1 Bt proteins on SPW larval mortality
The mortality of C. puncticollis larvae was significantly (l ≤ 0.001) higher on diet treated with hexadecylcaffeic acid or on Bt protein only than on untreated diet treated (Fig. 4). When hexadecylcaffeic acid was applied at 0.1 mg ml−1 together with Bt proteins in the diet, it caused a significantly higher mortality (76.7% P ≤ 0.001; Table 1) compared to when 0.1 mg ml−1 and Bt proteins were applied individually indicating an additive interaction of the two components (Fig. 4, Tables 1, 2). The mortality based on transformed data increased significantly when treatments were combined indicating an additive effect (Table 2).
Mean mortality (± SEM) of C. puncticollis larvae fed on diets treated with hexadecylcaffeic acid and Bt toxins. C16Cafflow; 0.01 mg ml−1 of hexadecylcaffeic acid treated diet: C16CafflowBt; 0.01 mg ml−1 hexadecylcaffeic acid and Bt-treated diet: C16Caffhigh; 0.1 mg ml−1 hexadecylcaffeic acid-treated diet: C16CaffhighBt; 0.1 mg ml−1 and Bt-treated diet: Bt; Bt-treated diet
Larval mortality caused by hexadecylcoumaric acid esters and Bt proteins was higher than on the untreated diet (Fig. 5). Treating the diet with 0.1 mg ml−1 hexadecylcoumaric acid esters caused 50% mortality in larvae of C. puncticollis (Fig. 5). A combination of 0.1 mg ml−1 of hexadecylcoumaric acid and Bt protein in the diet increased the mortality to 76.7% for C. puncticollis (Fig. 5). The mortality of the combination with protein was significantly higher (P ≤ 0.05) than when 0.1 mg ml−1 hexadecylcoumaric acid and Bt protein were tested separately in the diet indicating an additive interaction (Table 2).
Mean mortality (± SEM) of C. puncticollis larvae fed on diets treated with hexadecylcoumaric acid and Bt toxin. C16Coumlow; 0.01 mg ml−1 of hexadecylcoumaric acid treated diet: C16CoumlowBt; 0.01 ng/µL hexadecylcoumaric acid and Bt-treated diet: C16Coumhigh; 0.1 mg ml−1 hexadecylcoumaric acid-treated diet: C16CoumhighBt; 0.1 mg ml−1 hexadecylcoumaric acid and Bt-treated diet: Bt; Bt-treated diet
There was no significant difference (P ≥ 0.05) between the higher and lower concentrations of hydroxycinnamic acid esters on C. puncticollis larval mortality; diet treated with hexadecylcoumaric acid at 0.01 mg ml−1 caused 43.3% larval mortality (Fig. 5). The interaction between 0.01 mg ml−1 of hexadecylcaffeic acid ester combined with Bt protein in the diet was highly significant (P ≤ 0.001, Table 1) and the mortality significantly higher than from individual treatments. Combining Bt with hexadecylcoumaric acid more than doubled the mean mortality of C. puncticollis larvae indicating an additive effect (Table 2).
Diet treated with octadecylcoumaric acid at 0.01 mg ml−1 significantly (P ≤ 0.001, Table 1) affected C. puncticollis larval survival as did Bt proteins incorporated in the diet alone compared to the control (Fig. 6), while diet treated with 0.1 mg ml−1 of octadecylcoumaric acid caused an even higher larval mortality of 67% in C. puncticollis (Fig. 6). However, diet treated with 0.1 mg ml−1 of octadecylcoumaric acid and Bt proteins combined increased mortality significantly (P ≤ 0.001, Table 1) to 80% in C. puncticollis larvae (Fig. 6). The application of 0.01 mg ml−1 octadecylcoumaric acid in the diet also affected larval survival resulting in 50% mortality in C. puncticollis larvae (Fig. 6). The larval mortality on diets containing a combination of octadecylcoumaric acid at 0.01 mg ml−1, and Bt proteins was 67% and significantly (P ≤ 0.001, Table 1) higher than mortality from diets treated separately with these components (Fig. 6). The combination of Bt-proteins and octadecylcoumaric acid esters increased the mortality recorded by individual treatments applied in the diet singly indicating an additive interaction of the two treatments (Table 2).
Mean mortality (± SEM) of C. puncticollis larvae feeding on diets treated with octadecylcoumaric acid and Bt-toxins. C18Coumlow; 0.01 mg ml−1 of octadecylcoumaric acid treated diet: C18CoumlowBt; 0.01 ng/µL octadecylcoumaric acid and Bt-treated diet: C18Coumhigh; 0.1 mg ml−1 octadecylcoumaric acid-treated diet: C18CoumhighBt; 0.1 mg ml−1 octadecylcoumaric acid and Bt-treated diet: Bt; Bt-treated diet
Mortality of C. puncticollis larvae was significantly (P < 0.001) higher on the diet treated with the 5-O-caffeoylquinic acid than on untreated controls (Fig. 7). There was a significant difference (P ≤ 0.001, Table 1) between 0.1 and 0.01 mg ml−1 of 5-O-caffeoylquinic acid on mortality of C. puncticollis larvae. The mortality of larvae on diets containing Bt-proteins and 5-O-caffeoylquinic acid was greater than the mortality caused by treatments with individual components indicating an additive interaction (Fig. 7 and Table 2).
Mean mortality (± SEM) of C. puncticollis larvae feeding on diets treated with 5-O-caffeoylquinic acid and Bt toxins. Chlolow; 0.01 mg ml−1 of 5-O-caffeoylquinic acid treated diet: ChlolowBt; 0.01 mg ml−1 5-O-caffeoylquinic acid and Bt-treated diet: Chlohigh; 0.1 mg ml−1 5-O-caffeoylquinic acid-treated diet: ChlohighBt; 0.1 mg ml−1 5-O-caffeoylquinic acid and Bt-treated diet: Bt; Bt-treated diet
Discussion
Cinnamoyl esters that occur naturally in the roots of resistant sweetpotato reduced feeding and egg-laying by adults of both C. puncticollis and C. brunneus as determined by significantly lower feeding holes, reduced faecal production and fewer eggs laid on treated root cores than on the untreated root cores. The effect was typically dose dependent concurring with previous work suggesting that the concentration of these compounds at the root surface was critical in their function as resistance factors (Anyanga et al. 2013) and in their toxicity to larvae (Stevenson et al. 2009). Higher numbers of feeding holes recorded for C. brunneus compared to C. puncticollis on the root core treated with similar concentrations of octadecylcaffeic acid, octadecylcoumaric acid and 5-O-caffeoylquininc acid esters suggested that the sensitivity of the two pest species to these compounds differed, so this should be considered in the development and deployment of resistant varieties. That said, overall the effects of compounds on feeding were similar in both species on root core treated with heptadecylcaffeic acid. These naturally occurring compounds could contribute to resistance in this crop against adults and may be useful traits to monitor in resistance breeding programs since they also segregate with resistance (Otema et al. 2017). Since there are several components contributing to the resistance, this may also have benefits in limiting the development of resistance. In related work, Akhtar et al. (2008) reported that plants with phytochemicals that have similar activities but occurring as several different but related structures can reduce development of insect resistance to such defence mechanisms. This concurs with earlier predictions where coumaroyl esters were previously associated with Cylas resistance although these compounds were not directly tested against weevils (Snook et al. 1994). Caffeoyl quinic acids which have previously been associated with defence in sweetpotato to Diabrotica spp. have been reported (Jackson and Bohac 2006), suggesting a quantitative resistance to this insect that could be related to the effect reported in the present work with Cylas spp. since both studies presented adults with root surfaces and showed a similar effect.
Perhaps the most significant effects of these compounds on adults was on oviposition. There were no eggs laid by either pest species on root cores treated with 0.1 mg ml−1 of hydroxycinnamic acid esters. Thus, developing sweetpotato varieties with higher concentrations of these compounds through breeding, would reduce feeding by adults and also hinder egg laying with benefits of reduced cortical damage. Breeding for sweetpotato varieties with high levels of hydroxycinnamic acid esters on the root surface may be an effective way of reducing successful colonization at the first point of contact with the pest and reduce successful infestation but will unlikely exclude larvae completely.
Previous work has shown that the chemistry of the cortical tissues of resistant and susceptible clones does not differ and therefore larvae that emerge from eggs laid by adults that avoided surface resistance mechanisms could go on to colonise the root (Anyanga et al. 2013). Thus, to prevent successful colonization by SP weevils an additional mechanism would be required to supplement the activity of the cinnamic acid esters at the root surface. This could be provided through transformation of plants to produce Bt proteins which are known to be biologically active against SPW larvae (Ekobu et al. 2010). Recent work by Rukarwa et al. (2013) transformed sweetpotato with Bt protein and developed ten transgenic events expressing Cry7Aa1, Cry3Ca1 and ET33-34 proteins for sweetpotato weevil resistance, but none of those events provided effective insect pest control of C. puncticollis due to poor expression in the plant. The transformed plant was a weevil susceptible variety; thus it is possible if expressed in a resistant variety that Bt protein could interact with bioactive plant compounds that confer resistance to enhance their activity but until now their interactions with these naturally occurring compounds remain untested. If the expression could be enhanced, then in combination with other factors the activity could be complementary which has been demonstrated by this study.
Here we report that one of the toxic Bt proteins used by Rukarwa et al. (2013), Cry7Aa1, was biologically active against larvae of an African Cylas species concurring with earlier work (Ekobu et al. 2010) and importantly that mortality caused by the Bt protein in combination with the naturally occurring hydroxycinnamic acid esters in the root caused an increased mortality greater than the individual components at a level expected for an additive effect. The concentration of Bt protein we used in bioassays was that which caused mortality of SPW larvae using a protocol developed by Ekobu et al. (2010) and by Rukarwa et al (2013). We have shown that the effect of combining two treatments (Bt protein and HCAs) caused greater mortality than the sum of the two individual treatments. We also found that using a higher concentration of Bt protein here increased the mortality when both treatments were provided together than the same treatments at the same concentration when provided alone indicating an additive interaction. Specifically, there was higher mortality in diets where Bt proteins were incorporated in combination with hexadecylcaffeic acid compared to the diet treated with either Bt proteins or hexadecylcaffeic acid. These data indicate that Bt proteins and hydroxycinnamic acid increased the mortality and in fact doubled the effect of the individual treatments and may improve efficacy of Bt protein in transformed roots on larval mortality in combination with hexadecylcaffeic acid suggesting both mechanisms influence food acquisition. Our previous data (Amoabeng et al. 2013) suggest that the effects of these plant surface compounds are to reduce feeding. Thus, when presented in combination with a Bt protein the plant compounds could reduce consumption of the toxic protein, thereby reducing the effects of the protein but our data indicate an additive interaction of the toxic effect of the two mechanisms in combination (Table 1).
The activity of caffeic acid esters is likely due to the dihydroxy phenolic moiety which binds covalently to proteins so can interfere with digestive processes or reduce availability of proteins in food and thus reduces insect development (Stevenson et al. 1993; Duffey and Felton, 1991). Gill et al. (1992) indicated that Bt protein also acts by interfering with insect digestion by creating holes in the insect larval gut membrane leading to leakage of gut content and eventually the death of susceptible insects. It is now clear that these two mechanisms could complement one another other and if the impact of one is already high enhancing that effect with a different mechanism that also inhibits nutrient uptake could provide a more resilient mechanism reducing the scope for resistance in pest insects.
Plant compounds may be antagonistic for Bt efficacy by reducing feeding. For example, Navon et al. (1991) reported that leaf feeding by the larvae of Heliothis virescens and larval survival and weights decreased with an increase in Bt concentration. Antifeedant effects of acid exudates reduced food consumption and hence the dose and efficacy of Bt sprays on insect resistance. Our previous data show that compounds that confer resistance in sweetpotato are deterrents to adults (Anyanga et al. 2013) so may also reduce consumption of Bt protein by weevil larvae. Surekha et al. (2011) reported that the biological activity of Bt was lower on artificial diets with leaf or pod powder of resistant chickpea genotypes, which might also be explained because of reduced consumption of Bt protein due to the antifeedant effects of acid exudates in the chickpea. It may also be due to the interaction of biochemical constituents in chickpea with Bt protein. Nevertheless, larval survival, larval and pupal weights and adult emergence were significantly lower on diets with leaf or pod powder of the H. armigera resistant genotypes with Bt protein than on susceptible control. Chickpea genotypes with resistance to H. armigera acted in concert with Bt protein to cause adverse effects on the survival and development of this insect. In this study, there was additive effect of hydroxycinnamic acid esters and Bt and that there was no antagonistic interaction of the resistance compounds. Bt proteins and hydroxycinnamic acids both cause mortality in SPW larvae and in combination increased mortality as is expected for an additive effect. We suggest that Bt strains that can be expressed effectively in the root cortex of sweetpotato could enhance resistance mediated by hydroxycinnamic acid esters, and should be explored as a trait that breeders can exploit for biorational pest management of sweetpotato weevils (Otema et al. 2017; Yada et al. 2017), but future development of multiple component resistance should consider the potential for interactions of different mechanism that reduce efficacy.
Author contributions
MO, GS, RM and PS conceived the research. MO conducted the experiments and drafted the MS with PS. DF developed the dilution protocol. All authors analyzed data and edited the manuscript.
References
Akhtar Y, Yeoung YR, Isman MB (2008) Comparative bioactivity of selected extracts from Meliciae and some commercial botanical insecticides against two noctuid caterpillars, Trichloplusia and Pseudaletia unipuncta. Phytochem Rev 7:77–88
Anyanga MO, Muyinza H, Talwana H, Hall DR, Farman DI, Ssemakula GN, Mwanga ROM, Stevenson PC (2013) Resistance to the Weevils Cylas puncticollis and Cylas brunneus Conferred by Sweetpotato Root Surface Compounds. J Agric and Food Chem 61:8141–8147
Bravo A, Gill S, Soberón M (2007) Mode of action of Bacillus thuringiensis Cry and Cytotoxins and their potential for insect control. Toxicon 49:423–435
Ekobu M, Solera M, Kyamanywa S, Mwanga ROM, Odongo B, Ghislain M, Moar WJ (2010) Toxicity of seven Bacillus thuringiensis Cry Proteins against Cylas puncticollis and Cylas brunneus (Coleoptera: Brentidae) using a novel artificial diet. J Econ Entomol 103:1493–1502
EPA. (2018). Current and Previously Registered Section 3 Plant-Incorporated Protectant (PIP) registrations. https://www.epa.gov/ingredients-used-pesticide-products/current-previously-registered-section-3-plant-incorporated. Accessed 12 May 2020
Fernández-Grandon M, Harte S, Ewany J, Bray DP, Stevenson PC (2020) Additive effect of botanical insecticide and entomopathogenic fungi on pest mortality and the behavioral response of its natural enemy. Plants 9:e173
Fite T, Getu E, Sori W (2014) Integrated Management of Sweetpotato Weevil: Cylas puncticollis (Boheman) (Coleoptera: Curculionidae) in Eastern Ethiopia. J Entomol 11:225–237
Gill SS, Cowless EA, Pientrantonio PV (1992) The mode of action of Bacillus thuringiensis endotoxins. Ann Rev Entomol 37:615–636
Kiiza B, Mwanga ROM, Kisembo L, Kreuze J, Labarta R, Ghislain M (2009) Analysis of economic implications of biotech sweetpotato in the Great Lakes Region to control weevil and virus disease damage. Uganda Country Report.
Laurie RN, Laurie SM, Du Ploy CP, Finnie JF and Staden J Van (2015) Yield of drought-stressed sweetpotato in relation to canopy cover, stem length and stomatal conductance. J Agric Sci 7:201–214
Jackson DM, Bohac JR (2006) Improved dry-fleshed sweetpotato genotypes resistant to insect pests. J Econ Entomol 99:1877–1883
Loebenstein G (2009) Origin, distribution and economic importance. In: Loebenstein G, Thottappilly G (eds) The sweetpotato. Springer, New York
Moran R, Garcia R, Lopez A, Zaldua Z, Mena J, Garcia M, Armas R, Somonte D, Rodriguez J, Gomez M, Pimentel E (1998) Transgenic sweetpotato carrying the delta-endotoxin gene from Bacillus thuringiensis var tenebrionis. Plant Sci 139:175–184
Navon A, Hare JD, Federici BA (1991) Interactions among Heliothis virescens larvae, cotton condensed tannin and the Cry1A(c) δ-endotoxin of Bacillus thuringiensis. J Chem Ecol 19:2485–2499
Nottingham SF, Kays SJ (2002) Sweetpotato weevil control Acta Hort 583:155–161
Otema AM, Yada B, Yencho GC, Ssemakula GN, Alajo A, Farman DI, Mwanga ROM, Stevenson PC (2017) Segregation of Hydroxycinnamic Acid Esters Mediating Sweetpotato Weevil Resistance in Storage Roots of Sweetpotato. Front Plant Sci 8:1011
Pandey G (2009) Acute toxicity of ipomeamarone, a phytotoxin isolated from the injured sweetpotato. Pharmacognosy Magazine 4:89–92
Placide R, Shimelis H, Laing M, Gahakwa D (2013) Physiological mechanisms and conventional breeding of sweet potato (Ipomoea batatas (L) Lam) to drought-tolerance. Afr J Agric Res 8:1837–1846
Rees D, Van Oirschot QEA, Kapinga RE, Mtunda K, Chilosa D, Mbilinyi LB, Rwiza EJ, Kilima M, Kiozya H, Amour R, Ndondi T, Chottah M, Mayona CM, Mende D, Tomlins KI, Aked J, Carey EE (2003) Extending root shelf-life during marketing by cultivar selection. In: Rees D, Quirien O, Kapinga R (eds) SweetPotato postharvest assessment. Experiences from East Africa. University of Greenwich, London
Rukarwa RJ, Prentice K, Ormachea M, Kreuz JF, Tovar J, Mukasa SB, Ssemakula G, Mwanga ROM, Ghislain M (2013) Evaluation of bioassays for testing Bt sweetpotato events against sweetpotato weevils. African Crop Sci J 21:235–244
Sato K, Uritani I (1981) Characterization of the terpene-inducing factor isolated from the larvae of the sweetpotato weevil, Cylas formicarius Fabricius (Coleoptera: Brentidae). Appl Entomol Zool 16:103–112
Smit NEJM (1997) Integrated pest management for sweetpotato in Eastern Africa. Wageningen, Grayfish Service Centrum Van Gils B.V., pp 2–117
Snook ME, Data ES, Kays SJ (1994) Characterization and quantification of hexadecyl, octadecyl and eicosyl esters of P-coumaric acid in the vine and root latex of sweetpotato (Ipomoea batatas (L.) Lam.). J Agric Fd Chem 42:2589–2595
Stathers TE, Rees D, Kabi S, Mbilinyi L, Smit N, Kiozya H, Jeremiah S, Nyango A, Jeffries D (2003) Sweetpotato infestation by Cylas spp. In East Africa. I. Cultivar differences in field infestation and the role of plant factors. Intl J Pest Manage 49:131–140
Stathers TE, Rees D, Nyango A, Kiozya H, Mbilinyi L, Jeremiah S, Kabi S, Smit N (2003) Sweetpotato infestation by Cylas spp. in East Africa: II. Investigating the role of root characteristics. Intl J Pest Manage 49:141–146
Stevenson PC, D’Cunha RF, Grzywacz D (2010) Inactivation of Baculovirus by Isoflavonoids on Chickpea (Cicer arietinum) Leaf Surfaces Reduces the Efficacy of Nucleopolyhedrovirus Against Helicoverpa armigera. J Chem Ecol 36:227–235
Stevenson PC, Muyinza H, Hall DR, Porter EA, Farman DI, Talwana H, Mwanga ROM (2009) Chemical basis for resistance in sweetpotato Ipomoea batatas to the sweetpotato weevil Cylas puncticollis. Pure Appl Chem 81:141–151
Stevenson PC, Anderson JA, Blaney WM, Simmonds MSJ (1993) Developmental inhibition of Spodoptera litura larvae by a novel caffeoylquinic acid from the 26 wild groundnut Arachis paraguariensis (Chod et Hassl). J Chem Ecol 19(12):2917–2933
Storer NP, Babcock JM, Edwards JM (2006) Field measures of western corn rootworm (Coleoptera: Chrysomelidae) mortality caused by Cry34/35Ab1 proteins expressed in maize event 59122 and implications for trait durability. J Econ Entomol 99:1381–1387
Surekha Devi V, Sharma HC, Arjuna Rao P (2011) Interaction between host plant resistance and biological activity of Bacillus thuringiensis in managing the pod borer Helicoverpa armigera in chickpea. Crop Prot 30:962–969
Uritani L, Saito T, Honda H, Kim WK (1975) Induction of furano-terpenoids in sweetpotato roots by larval components of the sweetpotato weevils. Agric Biol Chem 37:1857–1862
Vaughn T, Cavato T, Brar G, Coombe T, DeGooyer T, Ford S, Groth M, Howe A, Johnson S, Kolacz K, Pilcher C, Purcell J, Romano C, English L, Pershing J (2005) A method of controlling corn rootworm feeding using a Bacillus thuringiensis protein expressed in transgenic maize. Crop Sci 45:931–938
Woolfe JA (1992) Sweetpotato, an Untapped Food Resource. Cambridge University Press and the International Potato Centre (CIP), Cambridge, UK
Yada B, Alajo A, Ssemakula GN, Brown-Guedira G, Otema MA, Stevenson PC, Mwanga ROM, Yencho GC (2017) Identification of simple sequence repeat markers for Sweetpotato weevil resistance. Euphytica 213:129
Acknowledgement
The authors thank Professor W.J. Moar, Auburn University, USA (now at Monsanto, Missouri), for providing Bt protein Cry7Aa1 and Professor David Hall (Natural Resources Institute (NRI) for providing hexadecylcaffeic acid and hexadecyl and octadecylcoumaric acid used in the study.
Funding
This study was funded by McKnight Foundation Collaborative Crop Research Project no. 08–019 on sweetpotato breeding to ROM and PCS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Communicated by M. Traugott.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Anyanga, M.O., Farman, D.I., Ssemakula, G.N. et al. Effects of hydroxycinnamic acid esters on sweetpotato weevil feeding and oviposition and interactions with Bacillus thuringiensis proteins. J Pest Sci 94, 783–794 (2021). https://doi.org/10.1007/s10340-020-01297-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-020-01297-5