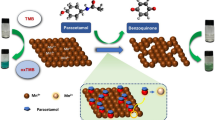
Abstract
Methionine and paracetamol are combined in dosage forms due to the ability of methionine to compensate the deficiency in glutathione in hepatic patients. In this work, an innovative simple time-saving green RP-HPLC method using glycerol as a solely green mobile phase with water for the first time was developed for the simultaneous determination of methionine and paracetamol in their standard and in Hepamol® tablets. The chromatographic conditions were optimized using factorial design with the aid of Minitab 17® Software. The method was performed on a C18 column at 38 °C, and a mobile phase consisting of glycerol and phosphate buffer (pH 2.4) (40:60, v/v), using diode array detector at 210 nm. The mixture was separated in 5 min. The developed method was validated in accordance with ICH requirement over linearity ranges of 10–90 μg/mL for both drugs, and LODs were 3.33 μg/mL for both methionine and paracetamol. Glycerol has high safety, low UV cut-off point, low flammability, and its viscosity can be manipulated when diluted with water. The procedure was compared to the reported reversed phase liquid chromatography method in the terms of their greenness with the green analytical procedure index and the analytical eco-scale. This work breaks new ground for scientists to use glycerol in greener RP-HPLC applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Paracetamol remains one of the oldest and most commonly used central analgesics and antipyretics to date [1]. It alleviates pain by increasing the human pain threshold through the reduction of prostaglandin production in the brain and spinal cord [2]. Additionally, it has been considered a primary treatment for COVID-19 symptoms [3]. Severe cases of COVID-19 lead to a depletion of glutathione levels in the human body [4]. Several hypotheses have been proposed to explain the relationship between paracetamol use and glutathione deficiency, which may have adverse effects on hepatitis patients [5, 6].
Methionine, an essential amino acid found in many dietary supplements, plays a significant role in cell functions and in managing liver disorders. It is also known to help protect against paracetamol poisoning when co-formulated with paracetamol in a single dosage form. This combination is particularly beneficial for patients at risk of glutathione depletion, such as hepatitis patients and those with severe COVID-19 symptoms [7,8,9]. The chemical structures of methionine and paracetamol are illustrated in Fig. 1.
Numerous pharmaceutical companies worldwide routinely analyze paracetamol in their quality control (QC) laboratories, either as a raw ingredient or a finished pharmaceutical product. Consequently, several analytical methods have been developed for paracetamol determination in various pharmaceutical forms and biological fluids [2, 10]. Some methods, such as those by Ahmed et al. [11], Salih et al. [12], Kohler et al. [13], and Ibrahim [14], employ hazardous organic modifiers in the mobile phase, while others, like Ibrahim et al. [9], Abdel Aleem et al. [15], Riley et al. [16], and Kalmár et al. [17], utilize gradient programs. Hazai et al. [18] and Hewala et al. [19] opt for two mobile phase systems. These methods, however, may be environmentally unfriendly, costly, and involve hazardous organic solvents and complex techniques like dual-gradient techniques.
Some methods have been developed for the determination of methionine co-formulated with paracetamol using a C18 column. Fares et al. [20] suggest that the gradient mode is suitable for their method, whereas Ibrahim et al. [9] employ a dual-mode gradient program and a ternary mobile phase with non-eco-friendly solvents. One recent method [21] employs a peak enhancement technique to simultaneously elute methionine and paracetamol, using di-ethylamine as an organic modifier—a toxic and highly flammable substance—in HILIC mode with a mobile phase consisting of large amounts of hazardous conventional solvents.
High-performance liquid chromatography (HPLC) stands as the most widely used technique in analysis and bioanalysis [2]. Despite its remarkable sensitivity, precision, accuracy, and ease of use, HPLC generates a significant amount of waste daily and contributes to environmental pollution. Anastas and Warner's fifth principle of green chemistry [22, 23], which emphasizes the elimination of hazardous organic solvents, directly applies to analytical chemistry and gives rise to the concept of green analytical chemistry [24]. Replacing flammable toxic solvents with eco-friendly alternatives represents a core principle of GAC [25,26,27]. The primary challenge in this context is striking a balance between improving result quality, analytical performance, and separation while enhancing environmental friendliness [28]. Glycerol has gained favor among scientists as a solvent due to its unique properties [29]. Glycerol is biodegradable, non-flammable, non-toxic, readily available, cost-effective, and safe [30].
Due to its intermediate polarity between water and other conventional RPLC organic solvents, glycerol provides fine-tuning capabilities for mixtures that may not separate well using other conventional RPLC organic solvents, as demonstrated in previous work by the author [31, 32].
The choice of a green mobile phase organic modifier is crucial in HPLC methods employing UV detection, primarily due to the lowest usable (cut-off) wavelength [33]. Glycerol, with its lower cut-off point at 207 nm compared to other explored green HPLC organic modifiers, such as acetone, ethyl acetate, and propylene carbonate [34], was previously overlooked in analytical chemistry due to its high viscosity. However, the viscosity of glycerol decreases significantly with the addition of water and changes in temperature [35]. The strong hydrogen bonding between glycerol and water leads to a volumetric contraction of glycerol when mixed with water, resulting in a noticeable reduction in glycerol viscosity and density [35]. Reported viscosity values for aqueous solutions of 40% (v/v) of acetonitrile, methanol, and glycerol at 40 °C are 0.65 cP, 1.11 cP, and 2.07 cP [36], respectively, under the same conditions as those employed in this developed method.
In this study, glycerol, as a green and safe organic solvent, replaces conventional organic solvents (acetonitrile and methanol) in a newly developed RP-HPLC method for the simultaneous determination of methionine and paracetamol. This substitution has no adverse environmental impact and results in improved peak shapes, opening the door for future RP-HPLC applications and applications in other liquid chromatography modes.
Experimental Methods
Chemicals
Methionine (MET, 99.1%) and Paracetamol (PC, 99.6%) were kindly supplied by Hikma Pharmaceuticals Co, 6th October, Giza, Egypt. Glycerol (purity 99%), and HPLC grade acetonitrile and methanol (> 99.9%; v/v) were purchased from Fisher pharmaceuticals (Fair Lawn, NJ). Potassium di-hydrogen phosphate and phosphoric acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). Mg stearate, stearic acid, sodium gluconate, aerosil 200 anhydride, sodium starch glycolate, and povidone were kindly supplied by Sigma Pharmaceutical Industries (Quesna, El Monofeya, Egypt).
Instrumentation and Software
The chromatographic separation was performed on a Dionex UltiMate 3000 HPLC (Thermo Scientific™, Dionex™, Sunnyvale, CA, USA) consisting of a quaternary pump (LPG-3400SD), on-line vacuum degasser (series 200), an autosampler (WPS-3000TSL), a column thermostat (TCC-3000SD), and a diode array detector (DAD-3000). Data acquisition and processing were performed by Chromeleon 7 software. Thermo Hypersil BDS C18 column (250 mm × 4.6 mm i.d., 5 µm particle size) was used throughout this study. The pH was measured using HANNA pH HI 110 benchtop pH-meter (Limena, Italy). Purified water was prepared with Millipore (Milford, USA) distillation apparatus and used for buffer preparation. The injection volume was 10 μL and detected by photo-diode array detector (DAD) at 210 nm. Minitab 17® Software (State College, PA) was used for method optimization by factorial design.
Stock and Working Solutions
Stock solutions of 1.0 mg/mL of MET and PC were prepared in distilled water and stored at 2 °C until use. Working solutions were prepared by transferring of 150 μL of MET and 750 μL of PC to 10 mL volumetric flasks and completing each flask to the volume with distilled water to get a final concentration of 15 μg/mL for MET and 75 μg/mL for PC.
Preparation of Mobile Phase
The mobile phase was directly pumped using one line of the pump through offline mixing. The eluent was prepared by mixing of 5 mmol. L−1 phosphate buffer and glycerol, pH 2.4, (200 mL glycerol in 500 mL of 5 mmol L−1 phosphate buffer, to prepare 40% (v/v) glycerol) and sonicated for 20 min.
Analytical Method Validation
The method was validated according to ICH Q2(R1) in terms of linearity, precision, accuracy and robustness [37]. The linearity was evaluated by the correlation coefficient (r) at concentrations ranging from 10 to 90 μg/mL of each analyte. The closeness of the assay results to the true value (the accuracy) was investigated by calculating %recoveries for MET and PC in triplicates at 30, 50, and 70 μg/mL. The intra-day precision test was performed by analyzing six replicates of samples in a single day, whereas the inter-day precision was assessed by analyzing binary mixtures of MET and PC in three consecutive days. The robustness was evaluated by screening the effect of small changes in various factors including small variations in the column temperature (± 2.0 °C), flow rate (± 0.20 mL/min) and glycerol concentration (mobile phase strength) (± 0.4%) using a Thermo Hypersil BDS C18 (250 mm × 4.6 mm, 5 µm) column.
Procedure of Dosage Form Analysis
Ten (Hepamol®) tablets were weighted, and quantity equivalent to 100 mg MET and 500 mg PC were transferred to a 100 mL volumetric flask, made up to volume, sonicated then filtrated through a 0.45 µm filter paper. The solution was then diluted with distilled water to obtain a series of three concentrations (μg/mL) in two replicates of MET and PC.
Results and Discussion
Because of the critical issue of using glycerol with high percentages and in order to be sure of the HPLC pump safety, the backpressure was correlated in previous studies to the flow rate of the mobile phase in HPLC with columns of (Particle’s sizes = 5 and 3. 5 µm) and UPLC of column of (Particle size = 1.7 µm), the back—pressure did not exceed limits in HPLC (415 bar) nor in UPLC (1250) bar, using glycerol as a mobile phase modifier [32]. The effect of increasing the glycerol/water ratio on the backpressure was studied also in previous studies and compared with acetonitrile as a standard organic modifier at 8 different temperatures. Increasing the amount of glycerol (%, v/v) in the mobile phase from 7 to 40% did not exceed the operating limits (700 bar) [31]. The backpressure was approximately 250 bar, which amounted to less than half of the pump’s maximum capacity. This pressure level falls within the optimal operating range of the instrument.
Application of Glycerol for Separation of MET and PC
The concomitant effects of % glycerol in mobile phase composition, the flow rate, and the column oven temperature were the factors involved in preliminary studies carried out through the optimization process by the injection of a sample solution containing MET and PC through three chromatographic conditions followed by a full factorial design. The three chromatographic conditions that were involved in the preliminary studies of the optimization process started with a percentage of 35% (v/v) glycerol, 38 °C and 1.2 mL/min, which resulted in a higher resolution (Rs) between paracetamol peak and the peak of methionine but with a lower number of theoretical plates (N) and a longer run time Figure S1a, then 35% (v/v) glycerol, 1.5 mL/min and 40 °C were tried for the separation of the mixture and resulted into more baseline noise and a lower number of theoretical plates (N) Figure S1b. Finally, flow rate of 1.5 mL/min, higher temperature of (40 °C), and percentage of glycerol of 40% (v/v) were tried throughout the optimization process, and lower resolution (Rs), lower asymmetrical factors (As), and lower number of theoretical plates (N) were noticed (Figure S1c). The constraints were set for each variable, based on the preliminary studies (Table S1), then Minitab 17® software was used for method optimization based on the run time and the asymmetry factor. The model was set to minimize run time, and to attain an asymmetry factor of one. A set of eight runs suggested by the software were performed, and the optimization parameters were used to calculate the desirability values (d) which was 0.8. Fig. S2 shows the interaction plot for the run time and the desirability curve. The optimum chromatographic conditions that were desired by the factorial design study suggested to adjust flow rate at 1.5 mL/min and a glycerol concentration of 40% at 38 °C. The final chromatographic conditions were at isocratic elution mode with a (Thermo Hypersil BDS C18 (250 mm × 4.6 mm, 5 µm) column at 38 °C and a mobile phase composed of glycerol: potassium di-hydrogen phosphate buffer (5 mM, pH 2.4) (40:60, v/v), detected by DAD at 210 nm, flow rate was 1.5 mL/min, injection volume: 10 µL and the analyte concentration was 15 μg/mL of MET and 75 μg/mL of PC.
Methionine is a compound that has a weak chromophore. Therefore, HPLC analytical methods that employ C18 columns in the reversed phase mode for the determination of methionine are challenging, [38] due to the weak retention. Glycerol as an RP-HPLC organic solvent could be used as a mobile phase modifier to separate MET and PC in 5 min with good resolution, detection limits, and symmetric peak shapes in spite of the significantly different log as shown in Table S2. The chromatographic separation of MET and PC at the optimum conditions is shown in Fig. 2.
System Suitability
System suitability test is an essential parameter to confirm that the developed method meets the requirement for a reliable HPLC method. Different system suitability parameters, such as retention factor (K), resolution (Rs), asymmetry factor (As), number of theoretical plates (N), and selectivity factor (α), were measured to evaluate the performance of the developed method for determination of MET and PC. The retention time ranged between 2.5min and 5 min and the resolution had a value of 7.78 between methionine and paracetamol, which is acceptable according to the ICH guidelines. The mixture had an acceptable selectivity factor of 1.08. The peaks were symmetric, with an asymmetric factor ≤ 1.15. The number of theoretical plates was in the range 3035–4089, which shows good method efficiency. These results show that the developed method fits its purpose. The results of the system suitability study and the acceptance criteria are summarized in Table 1.
Validation
The developed method was validated according to the guidelines of the International Conference on Harmonization Q2 (R1) [37]. Method linearity, range, precision, accuracy, limit of quantitation, limit of detection, and robustness were studied.
Linearity
The linearity of the HPLC method for determination of MET and PC was evaluated by injection of a series of different concentrations of each drug. In this study, six concentrations were chosen, ranging between 10 and 90 μg/mL for MET and PC. Each concentration was repeated three times in order to detect the variation in peak area values between samples of the same concentration. A calibration curve was created by plotting concentrations against peak area and regression analysis was performed. The coefficients of determination (r2) were determined for MET and PC and found to be equal to 0.999. The regression parameters including slopes, intercepts, coefficients of determination, and linearity ranges are summarized in Table 2.
Detection (LOD) and Quantitation (LOQ) Limits
According to the International Conference on Harmonization (ICH) recommendations, limit of detection (LOD) and limit of quantitation (LOQ) were calculated to determine the smallest concentration of both drugs that can be detected and accurately quantified. The approach based on the standard deviation (SD) of the response and the slope was used for determining the detection and quantitation limits as the following equations:
where SD is the standard deviation of 10 blank injections. The results showed that the LODs were 3.33 μg/mL and 3.33 μg/mL, while the LOQs were 10.03 μg/mL and 10.03 μg/mL for MET and PC respectively.
Accuracy and Precision
Throughout the dosage form analysis, the excipients in pharmaceutical formulations do not interfere in the analysis of the two compounds in their pharmaceutical formulations. Accordingly, %Recoveries were calculated for MET and PC at triplicates to determine the closeness of the assay results to the true value (the accuracy). Each concentration was repeated three times for each drug in the µgmL−1 range and the average was used to calculate the %recovery for each drug. The results (Table 3) showed that the average %recoveries were in the range 99.65–99.89, which proves that the method is sufficiently accurate for pharmaceutical analysis.
The %RSD at the same concentration levels was calculated for the intra-day precision and inter-day precision studies by analyzing binary mixtures of MET and PC in the same day and in three consecutive days. As shown in Table 3, the %RSD did not exceed 0.67, which is adequate for dosage form analysis.
Robustness
Variation of the column temperature (± 2.0 °C), the flow rate (± 0.20 mL/min), and the glycerol concentration (mobile phase strength) (± 0.4%) did neither have significant effect on the asymmetry factors (As) of both drugs nor the resulted %recovery in HPLC method. The robustness study aimed to check the reliability of the developed method against small experimental variations. As shown in Table 4, the results proved that the developed HPLC method was robust against small changes in experimental conditions.
Application to Dosage Form Analysis
The proposed HPLC method was applied to the simultaneous determination of MET and PC in their formulation form (tablets), the dosage form Hepamol® containing 100 mg MET and 500 mg PC. Determination of two replicates of each equivalent concentration was performed. Satisfactory results were obtained for each compound in good agreement with label claims (Table 5).
Advantages of the Developed Glycerol Method Over Some Reported Methods
The developed glycerol method has been compared in the terms of its advantages and dis-advantages to other reported liquid chromatographic methods that are used to determine MET and PC in different forms. Table 6 Ibrahim et al. [9] found the determination of MET co-formulated with PC in pharmaceutical preparations using a C18 column, and a ternary mobile phase consisting of water/methanol/acetonitrile with dual-gradient complicated program, and the solvents used in this method are non-eco-friendly, with potential harms on operators and the environment.
Fares et al. [20] also assumed gradient mode suitable for the method implication and used a mobile phase consisting of triethylamine: methanol to determine the same binary mixture in the rat plasma with diode array detection while Hasan et al. [39] could measure methionine in tablets with a relatively lower percentage of hazardous organic solvents but with a detection limit three times higher than paracetamol and also shifted to the gradient mode. Binh et al. [21] could measure MET and PC in dosage form using di-ethylamine to enhance the peak shape of methionine due to its low chromophore and relatively high percentages of organic solvents as he shifted to another mode rather than reversed phase mode which is HILIC that necessitates using of very large amount of organic solvents. These reported methods, Table 6, showed some limitations when compared with the glycerol method such as the use of gradient elution programs instead of simple isocratic mode and a limitation in the greenness issue. The glycerol developed method covered most of these limitations that accompanied with the reported methods used for the determination of methionine and paracetamol simultaneously, the glycerol-based method exhibits lots of advantages as it is time-saving, economic, totally green, simple, sensitive, and selective method when compared to other reference methods.
Evaluation of the Method Greenness
Green analytical procedure index (GAPI) [40] and the Analytical Eco-Scale [41] are reliable parameters used for the qualitative and quantitative evaluations of the method greenness. The replacement of RP-HPLC hazardous conventional solvents, such as acetonitrile and methanol, with a green alternative like glycerol requires a greenness assessment for the overall used amounts of chemicals, waste, and the energy consumption through the method development. GAPI scale showed spread of green color in the pentagon of the developed method over the pentagon of the reference method (Fig. 3), while the GAPI of the reference method appears more yellow and red. This can be attributed to the high safety and the biodegradability of glycerol, which protects both operators and the environment. The analytical eco-scale subtracts the penalty points from a total of 100 according to the amount and the safety of chemicals, waste, and the energy consumption. The higher the score on the Analytical Eco-Scale, the greener the method [41]. As summarized in Table 7, the developed method that used glycerol as modifier is classified as “Excellent green method”, with less penalty points than the reference method.
Conclusion
To the best of our knowledge, the first fully validated RP-HPLC method that used glycerol as a green mobile phase organic modifier instead of other hazardous RP-HPLC conventional solvents for the simultaneously determination of MET and PC in their fixed dose combination was developed in this work. Glycerol showed significant advantages over other solvents such as better performance in terms of peak shapes and asymmetry, resolution, and lower limits of detection for measuring the two compounds within green metrics. The main limitation of glycerol is its high viscosity which can be easily manipulated with water and temperature control. This work may be revolutionizing for analytical chemistry scientists for more futuristic applications using glycerol as a green organic modifier in other modes of HPLC and in macromolecules analysis.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Abbreviations
- AES:
-
Analytical eco-scale
- DAD:
-
Diode array detector
- GAPI:
-
Green analytical procedure index
- HILIC:
-
Hydrophilic interaction liquid chromatography
- MET:
-
Methionine
- PC:
-
Paracetamol
- RP-HPLC:
-
Reversed phase high-performance liquid chromatography
References
Zealand N (2008) Review article paracetamol (acetaminophen): mechanisms of action. Pediatr Anesth 18:915–921. https://doi.org/10.1111/j.1460-9592.2008.02764.x
Naguib IA, Majed M, Albogami M et al (2023) Greenness assessment of HPLC analytical methods with common detectors for assay of paracetamol and related materials in drug products and biological fluids. Separations 10:283. https://doi.org/10.3390/separations10050283
Sestili P, Fimognari C (2020) Paracetamol-induced glutathione consumption: is there a link with severe COVID-19 illness? Front Pharmacol 11:1–7. https://doi.org/10.3389/fphar.2020.579944
Pandolfi S, Simonetti V, Ricevuti G, Chirumbolo S (2021) Paracetamol in the home treatment of early COVID-19 symptoms: a possible foe rather than a friend for elderly patients? J Med Virol 93:5704–5706. https://doi.org/10.1002/jmv.27158
Hayward KL, Powell EE, Irvine KM, Martin JH (2016) Can paracetamol (acetaminophen) be administered to patients with liver impairment? Br J Clin Pharmacol 81:210–222. https://doi.org/10.1111/bcp.12802
Sabaté M, Ibáñez L, Pérez E et al (2011) Paracetamol in therapeutic dosages and acute liver injury: causality assessment in a prospective case series. BMC Gastroenterol 11:80. https://doi.org/10.1186/1471-230X-11-80
Norman E, Dhairiwan R, Dargan PI, Wallace CI, Jones AL (2001) Paracetamol poisoning: can it be prevented? J R Coll Physicians Edinb 31(1):62–65
Buckley NA, Dawson AH (2016) Treatments for paracetamol poisoning. BMJ 2579:1–5. https://doi.org/10.1136/bmj.i2579
Ibrahim H, Hamdy AM, Merey HA, Saad AS (2021) Dual-mode gradient HPLC and TLC densitometry methods for the simultaneous determination of paracetamol and methionine in the presence of paracetamol impurities. J AOAC Int 104:975–982. https://doi.org/10.1093/jaoacint/qsab021
Montaseri H, Forbes PBC (2018) AC SC. Trends Anal Chem. https://doi.org/10.1016/j.trac.2018.08.023
Ahmed NR (2019) HPLC method for determination of paracetamol in pharmaceutical formulations and environmental water samples. Chem Sci Trans 8:237–243. https://doi.org/10.7598/cst2019.1486
Salih ME, Aqel A, Abdulkhair BY et al (2018) Simultaneous determination of paracetamol and chlorzoxazone in their combined pharmaceutical formulations by reversed-phase capillary liquid chromatography using a polymethacrylate monolithic column. J Chromatogr Sci 56:819–827. https://doi.org/10.1093/chromsci/bmy058
Primus TM, Kohler DJ, Furcolow CA et al (2004) Determination of acetaminophen residues in whole body brown treesnakes. J Liq Chromatogr Relat Technol 27:897–909. https://doi.org/10.1081/JLC-120029706
Ibrahim F, El-Enany N, El-Shaheny RN, Mikhail IE (2015) Development and validation of a new HPLC method for the simultaneous determination of paracetamol, ascorbic acid, and pseudoephedrine HCl in their co-formulated tablets. Application to in vitro dissolution testing. Anal Sci 31:943–947. https://doi.org/10.2116/analsci.31.943
Abdelaleem EA, Naguib IA, Hassan ES, Ali NW (2015) HPTLC and RP-HPLC methods for simultaneous determination of paracetamol and pamabrom in presence of their potential impurities. J Pharm Biomed Anal 114:22–27. https://doi.org/10.1016/j.jpba.2015.04.043
Kamberi M, Riley CM, Ma X, Huang CWC (2004) A validated, sensitive HPLC method for the determination of trace impurities in acetaminophen drug substance. J Pharm Biomed Anal 34:123–128. https://doi.org/10.1016/j.japna.2003.08.015
Kalmár É, Gyuricza A, Kunos-Tóth E et al (2014) Simultaneous quantification of paracetamol, acetylsalicylic acid and papaverine with a validated HPLC method. J Chromatogr Sci 52:1198–1203. https://doi.org/10.1093/chromsci/bmt177
Hazai E, Simon-Trompler E, Czira G et al (2002) New LC method using radioactivity detection for analysis of toxic metabolite of acetaminophen (paracetamol). Chromatographia 56:S75–S78
Hewala II (1994) High-performance liquid chromatographic and derivative difference spectrophotometric methods for the determination of acetaminophen and its degradation product in aged pharmaceutical formulations. Anal Lett 27:561–582. https://doi.org/10.1080/00032719408001095
Fares MY, Abdelwahab NS, Abdelrahman MM, Abdel-Rahman HM (2019) Determination of sofosbuvir with two co-administered drugs; paracetamol and DL-methionine by two chromatographic methods. Appl Pharmacokinet Study Bioanal 11:349–364. https://doi.org/10.4155/bio-2018-0191
Binh VN, Hue VTP, Ha PTT (2021) Peak shape enhancement using diethylamine in hydrophilic liquid interaction chromatography: Application in simultaneous determination of methionine and paracetamol. J Pharm Biomed Anal. https://doi.org/10.1016/j.jpba.2021.114214
Anastas PT, Warner JC (1998) Principles of green chemistry. Green chemistry: theory and practice. Oxford University Press, Oxford
Lee TD (2011) Introduction to modern liquid chromatography. J Am Soc Mass Spectrom 22:196. https://doi.org/10.1007/s13361-010-0021-8
Gałuszka A, Migaszewski Z, Namieśnik J (2013) The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TrAC Trends Anal Chem 50:78–84. https://doi.org/10.1016/j.trac.2013.04.010
Espino M, ÁngelesFernández M, Gomez FJV, Silva MF (2016) Natural designer solvents for greening analytical chemistry. TrAC Trends Anal Chem 76:126–136. https://doi.org/10.1016/j.trac.2015.11.006
Vian M, Breil C, Vernes L et al (2017) Green solvents for sample preparation in analytical chemistry. Curr Opin Green Sustain Chem 5:44–48. https://doi.org/10.1016/j.cogsc.2017.03.010
Tobiszewski M, Namieśnik J (2017) Greener organic solvents in analytical chemistry. Curr Opin Green Sustain Chem 5:1–4. https://doi.org/10.1016/j.cogsc.2017.03.002
Koel M, Kaljurand M (2006) Application of the principles of green chemistry in analytical chemistry. Pure Appl Chem 78:1993–2002
Mota CJA, Pinto BP, Claudio JA, de Lima AL (2017) Glycerol: a versatile renewable feedstock for the chemical industry. Springer, Cham. https://doi.org/10.1007/978-3-319-59375-3
Gu Y, Jérôme F (2010) Glycerol as a sustainable solvent for green chemistry. Green Chem 12:1127–1138. https://doi.org/10.1039/c001628d
Habib A, Mabrouk MM, Fekry M, Mansour FR (2021) Glycerol as a novel green mobile phase modifier for reversed phase liquid chromatography. Microchem J 169:106587. https://doi.org/10.1016/j.microc.2021.106587
Habib A, Mabrouk MM, Fekry M, Mansour FR (2022) Glycerol as a new mobile phase modifier for green liquid chromatographic determination of ascorbic acid and glutathione in pharmaceutical tablets. J Pharm Biomed Anal. https://doi.org/10.1016/j.jpba.2022.114870
Snyder LR, Kirkland JJ, Glajch JL (2012) Appendix II: properties of solvents used in HPLC. Pract HPLC Method Develop 3:721–728. https://doi.org/10.1002/9781118592014.app2
Yabré M, Ferey L, Somé IT, Gaudin K (2018) Greening reversed-phase liquid chromatography methods using alternative solvents for pharmaceutical analysis. Molecules. https://doi.org/10.3390/molecules23051065
Cristancho D, Delgado D, Martínez F et al (2011) Volumetric properties of glycerol + water mixtures at several temperatures and correlation with the Jouyban-Acree model. Revista Colombiana de Ciencias Químico Farmacéuticas 40:92–115
Glycerine Producers’ Association (1963) Physical properties of glycerine and its solutions. Glycerine Producers’ Association, New York
ICH (2005) Validation of analytical procedures: text and methodology Q2 (R1). In: International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, pp 1–13
Hroboňová K, Moravčík J, Lehotay J, Armstrong DW (2015) Determination of methionine enantiomers by HPLC on the cyclofructan chiral stationary phase. Anal Methods 7:4577–4582. https://doi.org/10.1039/c5ay00808e
Hasan HMA, Habib IH, Khatab AA (2017) RP-HPLC determination of paracetamol-containing components in quaternary and binary mixtures. Eur Chem Bull 6:330–335
Płotka-Wasylka J (2018) A new tool for the evaluation of the analytical procedure: green analytical procedure index. Talanta 181:204–209. https://doi.org/10.1016/j.talanta.2018.01.013
Gałuszka A, Migaszewski ZM, Konieczka P, Namieśnik J (2012) Analytical Eco-Scale for assessing the greenness of analytical procedures. TrAC Trends Anal Chem 37:61–72. https://doi.org/10.1016/j.trac.2012.03.013
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
AH: ideas, formulation of study goals; methodology and research leadership responsibility. MM: study design, methodology, conducting an investigation process. MF: conducted the practical work, participated in the results discussion, preparation and writing of the manuscript. FRM: design of methodology, presentation of the published work, coordination of research planning.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Habib, A., Mabrouk, M.M., Fekry, M. et al. Glycerol-Based Green RP-HPLC Method for Simultaneous Determination of Methionine and Paracetamol in Pharmaceutical Tablets. Chromatographia 86, 707–716 (2023). https://doi.org/10.1007/s10337-023-04283-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-023-04283-y