Abstract
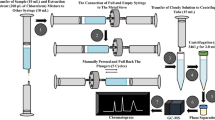
A simple, rapid, isocratic, and versatile liquid chromatographic method was developed for the simultaneous determination of bromhexine, guaifenesin, ambroxol, salbutamol/terbutaline, pseudoephedrine, triprolidine, and chlorpheniramine maleate in cough–cold syrups commonly marketed in Kenya. Separation was achieved using a Gemini® NX C18 column (250 × 4.6 mm, 5 μm) maintained at 40 °C and a mobile phase consisting of acetonitrile-0.25 M sodium hexanesulphonate-0.2 M ammonium acetate, and pH 3.0-water (35:4:10:51, % v/v/v/v) delivered at 1.0 mL min−1. The eluents were monitored by means of UV detection at 254 nm. During validation, the method satisfied the International Committee on Harmonization acceptance criteria for linearity, sensitivity, precision, accuracy, and robustness. The developed liquid chromatographic method was applied in the analysis of nine commercial samples obtained from Nairobi City County, Kenya. Extraction procedures were not applied during the assay of the samples, thus significantly shortening the analysis time.



Similar content being viewed by others
References
East African Pharmaceutical Loci (2015) A regional drug index for healthcare practitioners, 14th edn. Pharmaceutical Loci Publishers, Nairobi, pp 244–250
De Blasio F, Virchow JC, Polverino M, Zanasi A, Behrakis PK, Kilinç G, Balsamo R, De Danieli G, Lanata L (2011) Cough management: a practical approach. Cough 7:7
Katzung BG (2010) Basic and clinical pharmacology, 12th edn. McGraw-Hill, New York, p 563
Jongste JC, Shields MD (2003) Chronic cough in children. Thorax 58:998–1003
Ministry of Health (2016) Kenya Essential Medicines List 2016, Nairobi, p 63
Kigen G, Busakhala N, Ogaro F, Chesire E, Saat N, Too R, Nyandiko WA. Review of the ingredients contained in Over the Counter (OTC) cough syrup formulations in Kenya. Are they harmful to infants? PLoS One. 2015; 10(11)
Vervoort RJM, Ruyter E, Debets AJJ, Claessens HA, Cramers CA, de Jong JG (2001) Characterization of reversed-phase liquid chromatography stationary phases for the analysis of basic pharmaceuticals: eluent properties and comparison empirical test methods. J Chromatgr A 931:67–79
British Pharmacopoeia Commission (2015) British Pharmacopoeia. The Stationery Office, London
United States Pharmacopeial Convention ( 2015) United States Pharmacopeia 38 National Formulary 33. United States Pharmacopeial Convention Inc., Rockville, MD, Maryland
Porel A, Haty S, Kundu A (2011) Stability-indicating HPLC method for simultaneous determination of terbutaline sulphate, bromhexine hydrochloride and guaifenesin. Indian J Pharm Sci 73(1):46–56
Mallu UR, Bobbarala V, Penumajji S (2011) Analysis of cough and analgesic range of pharmaceutical active ingredients using RP-HPLC method. Int J Pharm Bio Sci 2(3):439–452
Jain V, Sharma MC (2016) Validated RP-HPLC method for determining the levels of bromhexine HCl, chlorpheniramine maleate, dextromethorphan HBr and guaiphenesin in their pharmaceutical dosage forms. J Taibah Univ Sci 10:38–45
Manassra A, Khamis M, el-Dakiky M, Abdel-Qader Z, Al–Rimawi F (2010) Simultaneous HPLC analysis of pseudoephedrine hydrochloride, codeine phosphate and triprolidine hydrochloride in liquid dosage forms. J Pharm Biomed Anal 51:991–993
Pai PNS, Rao GK, Murthy MS, Agarwal A, Puranik S (2009) Simultaneous determination of salbutamol sulphate and bromhexine hydrochloride in tablets by reversed phase liquid chromatography. Indian J Pharm Sci 71:53–55
Bhatia NM, Ganbavale SK, Bhatia MS, More HN, Kokil SU (2008) RP–HPLC and spectrophotometric estimation of ambroxol hydrochloride and cetirizine hydrochloride in combined dosage form. Indian J Pharm Sci 70:603–608
ICH harmonised tripartite guideline (Q2B) (R1) (2005) validation of analytical procedures: text and methodology. http://www.ich.org
Crowther JB (2001) Validation of pharmaceutical test methods. Sep Sci Technol 3:415–443
Shabir GA (2003) Validation of high-performance liquid chromatography methods for pharmaceutical analysis: understanding the differences and similarities between validation requirements of the US Food and Drug Administration, the US Pharmacopeia and the International Conference on Harmonization. J Chromatogr A 987(1):57–66
Law B, Houghton SJ, Ballard P (1998) An approach to the evaluation and comparison of reversed-phase high-performance liquid chromatography stationary phases. J Pharm Biomed Anal 17:443–453
Acknowledgments
The work presented in this paper was part of Master of Pharmacy thesis of PNM, University of Nairobi (2011). The authors wish to thank the University of Nairobi for financial support and the Management of the National Quality Control Laboratory for providing the necessary equipment to perform this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors of this manuscript have no financial and personal relationships with other people or organizations that could influence their work.
Rights and permissions
About this article
Cite this article
Njaria, P.M., Abuga, K.O., Kamau, F.N. et al. A Versatile HPLC Method for the Simultaneous Determination of Bromhexine, Guaifenesin, Ambroxol, Salbutamol/Terbutaline, Pseudoephedrine, Triprolidine, and Chlorpheniramine Maleate in Cough–Cold Syrups. Chromatographia 79, 1507–1514 (2016). https://doi.org/10.1007/s10337-016-3158-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-016-3158-1