Abstract
In monomorphic species, like in the Black-headed Gull, both sexes look alike in breeding plumage. With large sets of data on captured and photographed birds and using the Underhill–Zucchini moult model, we provided a detailed pattern of breeding plumage development in this species by age and sex. This study, similar to other studies, documented first adults with the initial stage of head moult at the beginning of January, yet the mean start date of nuptial moult in adults was the end of February. Half of adults acquired full breeding plumage about mid-March and almost all of them before mid-April. The start date of nuptial moult was more variable in immatures. The mean start of head moult in immatures was 19th April, which is 52 days later than in adults, and the majority, i.e., 70%, did not complete moult until the end of May. We showed for the first-time sex-dependent breeding plumage acquisition in monomorphic species. According to the Underhill–Zucchini moult model, males started to moult on average 7 days earlier than females and their moult lasted 7 days longer. Hence, the final date of completed head moult was the same in both sexes. A fully developed hood is an important part of the status signalling during pairing; therefore, completing the moult before mating is important for both sexes.
Zusammenfassung
Geschlechts- und altersabhängige Ausbildung des Brutgefieders bei einer monomorphen Art, der Lachmöwe Chroicocephalus ridibundus
Bei monomorphen Arten, wie der Lachmöwe, haben beide Geschlechter ein identisch aussehendes Brutgefieder. Anhand umfangreicher Datensätze von gefangenen und fotografierten Individuen sowie unter der Verwendung des Underhill-Zucchini-Mausermodells erstellten wir ein detailliertes Entwicklungsmuster vom Brutgefieder bei der Lachmöwe nach Alter und Geschlecht. Ähnlich wie in anderen Studien stellten wir fest, dass die ersten adulten Individuen Anfang Januar mit der Kopfgefiedermauser begannen, während der durchschnittliche Beginn der Brutmauser bei adulten Lachmöwen jedoch erst Ende Februar war. Die Hälfte der adulten Vögel bildete ihr Brutgefieder etwa Mitte März vollständig aus und fast alle beendeten die Mauser vor Mitte April. Der Beginn der Brutmauser zeigte eine höhere Variation bei immaturen Individuen. Der durchschnittliche Beginn der Kopfgefiedermauser bei immaturen Lachmöwen war der 19. April, was 52 Tage später als bei adulten Vögeln war, und die Mehrheit, d. h. 70%, schlossen die Mauser nicht bis Ende Mai ab. Wir konnten zum ersten Mal nachweisen, dass die Ausbildung des Brutgefieders bei einer monomorphen Art geschlechtsabhängig ist. Dem Underhill-Zucchini-Mausermodell zufolge begannen die Männchen durchschnittlich sieben Tage früher mit ihrer Mauser und mauserten insgesamt sieben Tage länger als die Weibchen. Somit ist der Zeitpunkt des Abschlusses der Kopfgefiedermauser bei beiden Geschlechtern gleich. Eine vollständig ausgebildete Kapuze ist ein wichtiger Bestandteil der Statussignalisierung während der Paarbindung, sodass der Abschlusszeitpunkt der Kopfgefiedermauser in männlichen und weiblichen Lachmöwen ähnlich ist. Andere Studien haben ebenfalls gezeigt, dass frühmausernde Männchen attraktiver für Weibchen sind, da sie eine bessere Fitness und somit einen höheren Reproduktionserfolg haben.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Birds moult at least once a year (Newton 2009), and this allows for the replacement of worn feathers and change in plumage colouration for mate choice or camouflage requirements (Beltran et al. 2018). In many bird species readiness for mating is associated with the development of breeding plumage, which is different from eclipse one (Butcher and Rohwer 1989; Hill 2006; Karlionova et al. 2008). Mate attraction and consequently increasing the chance for successful breeding (Andersson 1994) is a primary function of this plumage, as the degree of its development and the intensity of colours usually mirrors the condition of an individual and may be an honest signal of mate quality (Hill 1991, 2006; Estep et al. 2006; Minias et al. 2019). Breeding plumage acquisition must be accurately timed to environmental cues and synchronised within a population (Beltran et al. 2018). Smaller species of gulls, from the genus Chroicocephalus, may start to breed in their second calendar year of life, while larger species of the genus Larus start breeding later (Grant 1986). Immatures that develop colour plumage ornaments later and to a lesser extent are less likely to mate successfully (van der Jeugd and Blaakmeer 2001) and have lower breeding success than adults (Ryder 1975; Oring et al. 1991). It is assumed that delayed plumage maturation represents a strategy to maximize reproductive success as immatures cannot effectively compete with adults for breeding opportunities (Hawkins et al. 2012).
In the Black-headed Gull (Chroicocephalus ridibundus) both sexes look alike and a dark hood is the most conspicuous part of breeding plumage, both in males and females (Malling Olsen 2018). The Black-headed Gull starts to breed mainly in its third calendar year of life and at that time birds acquire full breeding plumage (Glutz von Blozheim and Bauer 1982). Although the pattern of acquisition of breeding plumage in this species has already been described, it is only a simple description of moult phenology with the rough assessment of start and end dates of head moult lacking more detailed analyses (Černy 1940; Franck and Epprecht 1959; Gloe 1983; Landman and Thaler 1984). The only published data on sex-related differences in breeding plumage acquisition concern black-headed gulls held in captivity, where the timing of a complete moult varied between the two pair members depending on the strength of their social preference (van Rhijn and Groothuis 1987).
The aim of the study was to investigate and determine possible age and sex differences in the phenology of breeding plumage development during nuptial moult of the Black-headed Gull. We used advanced methods based on the Underhill–Zucchini model with additional covariates (Underhill and Zucchini 1988; Underhill et al. 1990) to estimate the start, duration, and variation in the start date of acquiring breeding plumage in this species. Their use enables a far more detailed look at the moulting process and its progress by age and sex.
Materials and methods
We used two samples of birds. The first one was collected by taking photos with digital cameras with a telephoto lens to determine possible age-related differences in the time of nuptial moult. We took pictures of black-headed gulls that stayed along the municipal beach in Gdańsk (Gulf of Gdańsk, southern Baltic coast) and along the nearby Oliwski Stream in 1-week intervals between the beginning of January and the end of May 2020. All birds were aged according to plumage characteristics (Grant 1986) and two age categories were distinguished: birds in their first year of life (immatures) and older (adults). Observations of ringed individuals indicate that pseudoreplication in the sample of photographed birds is possible, particularly in the wintertime when black-headed gulls seem to be more stationary than during migration characterised by a high turnover rate. Therefore, the probability of sampling the same individuals is not constant over the studied period, but we do not have enough data to carry out a resampling analysis, as we did not count birds during the survey. The number of birds staying in the study area also varied greatly from around a hundred to about a thousand. Still particular care was taken to ensure that an individual was represented only once in the sample collected at a given date by carefully examining the photographs and eliminating individuals with identical head patterns from the daily sample. In total 1877 adults and 931 immatures were photographed. The second sample was used to determine possible sex-related differences in the time of nuptial moult and consisted of individuals captured for ringing with a loop trap and food bait (Meissner and Fischer 2017) in northern Poland, mainly in the Gulf of Gdańsk region between the beginning of January and the end of May in 1998–2005. In these gulls, we measured the total head length and bill depth at gonys with a dial calliper to the nearest 0.1 mm (Busse and Meissner 2015). Based on these measurements, we sexed birds using a discriminant function given by Palomares et al. (1997) for non-juvenile black-headed gulls. Due to the small sample size of immatures, we have considered only adults in this analysis, i.e., data on 587 adult males and 217 adult females. All caught birds were marked with ornithological metal rings and each individual was included in the analysis only once.
In both samples, we recorded head moult of an individual by assessing the percentage of new, blackish-brown feathers of the hood. We used eight categories to score moult: 0% for all old feathers, 1% for birds with single new feathers, four subsequent categories on a 20%-interval scale, 99% for almost complete breeding plumage, and 100% for all new feathers (Meissner et al. 2012, Fig. 1). However, as the majority of juveniles do not acquire full dark hood (Černy 1940; van Rhijn and Groothuis 1987), we cannot exclude that they finish the moult earlier, and the moult score observed is a measure of the number of dark feathers obtained rather than moult progress. When analysing the data collected in this study, we were not able to assess whether this was the case, so for simplicity, we used the term ‘moult score’ as a measure of moult progress also in juveniles. Moult assessment in photographed birds was carried out by one person (EC), while six bird ringers carried out moult assessment in captured birds. In both cases, pre-training was done to check for consistency in scoring in line with the most experienced person (WM). The probability of misjudging the category of moult score was very low due to the wide score intervals. To make the moult index linear, which is an assumption of the Underhill–Zucchini moult model, the recorded head moult scores were transformed to a scale of 0–1 (Underhill and Zucchini 1988; Underhill et al. 1990), so the final moult indices were as follows: 0% = 0, 1–20% = 0.1, 21–40% = 0.3, 41–60% = 0.5, 61–80% = 0.7, 81–99% = 0.9, 100% = 1. The Underhill–Zucchini model based on a likelihood approach allows estimating the mean start date of moult and variation in the start date, as well as moult duration, and is widely used in moult studies of different species (e.g., Serra et al. 2006; Remisiewicz et al. 2010; Machín et al. 2018; Mazur et al. 2021). For the analyses, we used the package moult 2.2.0 (Erni et al. 2013) in R 4.0.5 (R Core Team 2021). In this package, the moult index is a dependent variable and age, or sex are covariates. The start date, duration of the moult, and the standard deviation in the start date are reported as the results of modelling with an indication of which parameter is significantly different within sex or age categories.
To show the moult parameters of the Black-headed Gull we used default Data Type 2, which was representative of our sample and included individuals not yet moulting, in moult, and birds which have completed moult (Underhill and Zucchini 1988). However, we are aware that data were collected in two phenological periods. In winter, when black-headed gulls are mainly sedentary (MacKinnon and Coulson 1987; Spaans 2000) and during spring migration when migrants from the wintering areas located to the west and south–west stopover in the study area (Meissner 2003). Thus, our samples included individuals from different wintering locations, i.e., locally wintering birds and those only stopping in the study area during migration. We assume that the results we obtained are representative of black-headed gulls breeding in the vast area from north–west Russia, Finland, Baltic countries, and north-eastern Poland as birds wintering in the western part of Europe pass numerously in spring along the southern Baltic (Wernham et al. 2002; Bønløkke et al. 2006; Bairlein et al. 2014; Valkama et al. 2014). Whereas those originating from Sweden and countries to the south of Poland are much less numerous in the southern Baltic (Szinai 1998; Cepák et al. 2008; Fransson et al. 2008; Kralj et al. 2013). There are no differences in the timing of migration between adult males and females of the Black-headed Gull (Fijn et al. 2022). Hence, we assumed that migration phenology did not affect the results concerning possible differences in moult advancement between sexes.
To compare the moult timing of males and females of the Black-headed Gull and to determine the differences between adults and immatures, we used models with sex or age as a covariate affecting the mean start date, the standard deviation of the start date, and moult duration in various combinations. We compared these models using the Akaike information criterion (AIC), where the best-fitted model had the lowest AIC of all models, and the highest AIC weight (wAIC). The Akaike weights assess the relative support that a given model has from the data compared with the alternative models (Burnham and Anderson 2002). Only models with ΔAIC (the difference in AIC between the best-fitted and subsequent candidate models) lower than 3.0 were shown as they are considered to be similar in their ability to describe the data (Burnham and Anderson 2002). In the analyses, we counted the date as days since 1 January. To visualise temporal changes in breeding plumage acquisition over the study period, we used a generalized additive mode smoothing in R package ggplot2 (Wickham 2016). This allowed us to verify if the acquisition of head breeding plumage in the Black-headed Gull progresses approximately linearly over time.
Results
Age-related pattern of breeding plumage acquisition
On 5th January, when the first data were collected, adults with the initial sign of the development of breeding plumage on the head represented 10% of the observed birds, whereas the first immature individual showing the initial stage of head moult was documented on 2nd February. On 29th March, 53% of adult gulls had completed head moult. During the last field session on 31st May, only 30% of immatures had fully developed hood.
The best model with the lowest AIC value contained all parameters (mean start date, standard deviation of the start date, and moult duration) and its AIC weight was 0.995. Hence, we considered only this model. The mean duration of head moult in adults was 34 days and it was completed on average on the 1st of April. The mean start of head moult in immatures was estimated on 19th April, which is 52 days later than in adults. Moreover, the variation in the start date was twofold higher in immatures than in adults. The majority of immature gulls did not complete nuptial moult until the end of May when the migration period was almost finished. Hence, the duration of head moult in immatures estimated at 59 days might be biased, because it was calculated only for 28 individuals that acquired full breeding plumage and the estimated, final date of completed moult exceeded the end of May, i.e., studied period (Table 1). In the period of fast head moult progress, the moult index increased approximately linearly both in adult and immature gulls (Fig. 2). The sample size of adults rapidly declined in mid-April, when their migration was finished, which resulted in a much wider confidence interval (Fig. 2).
Sex-related pattern of breeding plumage acquisition
The best-fitted model indicating a significant effect of sex on the mean start of head moult and moult duration was supported by the data at least twice as well as the rest of the models. The standard deviation of the start date did not differ between the sexes (Table 2). According to this model, males started moult on average 7 days earlier than females and their moult lasted 7 days longer (Table 3, Fig. 3). Hence, the date of completed head moult was the same in both sexes. Similar to age-related moult pattern, the moult score increased linearly in both sexes during the period of fast head moult progress (Fig. 3).
Effect of the data collection method
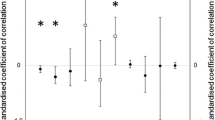
The mean start date of head moult in adult black-headed gulls was much earlier in captured birds than in the photographed ones (see Tables 1 and 3). However, significant differences occurred only in individuals with head moult index equal to 0.1 (1–20% of breeding plumage) (Fig. 4). Despite a 15 years difference between the collection of both samples, the overall pattern of acquiring subsequent moult stages remained similar in these two periods, except the initial phase.
Differences in the mean day of acquiring subsequent head moult index categories in adult black-headed gulls. White bars—captured gulls, grey bars—photographed gulls. Horizontal line—mean, rectangle—standard deviation, vertical line—range. Asterisk—differences statistically significant according to the Welch Two Sample t test at p < 0.05
Discussion
Data on the timing of acquiring breeding plumage reported in this study approximately fit those described many years ago for different sites in Europe. Half of adults acquire full breeding plumage about mid-March and almost all of them have breeding plumage fully developed before mid-April (Černy 1940; Franck and Epprecht 1959; Schwarzenbach 1960; Gloe 1983, 1984; Landman and Thaler 1984; Paterson 1993). Our study, similar to other studies (Černy 1940; Franck and Epprecht 1959; Gloe 1983; Landman and Thaler 1984), documented first adults with the initial stage of head moult at the beginning of January. However, single individuals with the hood partly or fully developed were reported as early as late November or early December (Landman and Thaler 1984; Paterson 1993).
We used a generalized additive mode smoothing to visualise moult progress over time, as there is no assumption about the type of modelled relationship in this model. In our study, moult progress in two age groups and in both sexes was approximately linear, which was also found in breeding plumage acquisition in the Little Stint (Calidris minuta) (Mazur et al. 2021) and in primary moult in a variety of species (i.e., Serra et al. 2006; Giunchi et al. 2008; Oschadleus and Underhill 2008; Remisiewicz et al. 2010; Machín et al. 2018).
In the Black-headed Gull the intensity and size of head markings are highly variable in winter plumage (van Dijk and Majoor 1995). The degree of plumage melanisation, controlled by testosterone (Bókony et al. 2008), may cause such high variability in winter head patterns and individuals with a larger number of dark feathers on their head may be classified as in moult. Birds with almost full breeding plumage are rarely seen in winter (Černy 1940; Franck and Epprecht 1959; Paterson 1993). They probably represent some abnormalities in the development of plumage, which were reported in various bird species (i.e., Rimmer and Tietz 2001; Cleere 2002), including melanism caused by a mutation in the extension (MC1R) and agouti (ASIP) genes (Mundy 2006; van Grouw 2017). Such individuals with many dark feathers on the head in their winter plumage possibly may be misidentified as birds in moult with partly developed breeding hoods. Consequently, the date of initiation of head moult in the Black-headed Gull is reported to be more variable than the date of moult completion (see Glutz von Blozheim and Bauer 1982). Similar to moult timing, the duration of nuptial head moult in adults estimated according to visual observations (photos) fit data collected 60 years ago when it was assessed as 45–56 days (Schwarzenbach 1960). However, in our study, the duration of head moult assessed from photos is shorter than moult duration calculated separately for males and females according to visual inspection of birds in the hand. Moreover, the mean start date is much earlier in birds inspected in the hand (cf. Tables 1 and 2). Single dark feathers are probably easier to find directly at a close distance than in photos, which may result in the observed differences when comparing data collected by two different methods. However, it cannot be excluded that the collection dates of both samples differing by more than 20 years may have influenced the reported onset of moult.
Spring migration of adult black-headed gulls in the vicinity of the study area starts in the first week of March and is finished at about the end of April (Meissner 2003). Immatures migrate later than adults—between mid-April and the second decade of May (Meissner 2003), but only some of them are observed in the breeding colonies and only a few (mainly females) breed in their second year of life (Glutz von Blozheim and Bauer 1982). In general, immature black-headed gulls start pre-breeding head moult between mid-March and mid-April, about 50 days later than adults (Černy 1940; Franck and Epprecht 1959, this study). However, the majority do not develop full breeding plumage (Černy 1940) and only 30% of immature birds observed in this study at the end of May had a full hood. Immature individuals observed in the breeding colonies in Poland usually had a dark hood with lots of white feathers (authors’ unpublished data). In black-headed gulls held in captivity the second calendar-year birds rarely acquired a fully dark head. If they did, however, it developed much later in the season than in their third calendar year (van Rhijn and Groothuis 1987).
At least in some avian species melanin-based plumage ornaments play a significant role in sexual selection (Yezerinac and Weatherhead 1997; Indykiewicz et al. 2017) and are associated with body condition predicting reproductive output (Crary and Rodewald 2012; Wiebe and Vitousek 2015). In the Black-headed Gull, the brownish-black hood in males and females is genuinely monomorphic with no differences in their size between sexes. However, the size of the brownish-black hood seems to be an honest signal of individual quality in males and females and may help in mutual mate choice in this species (Minias et al. 2019). Just after arrival at the breeding site or during the late stage of migration, the Black-headed Gull exhibits a range of ritualistic behaviours associated with pairing including characteristic postures, i.e., the head-flagging, upright posture, forward posture, where the head is extended towards the potential mate (Tinbergen and Moynihan 1952; Moynihan 1957). Similar behaviour was described in other gulls having dark hood in breeding plumage. Especially, the head-flagging is present in so-called ‘hooded gulls’ (Noble and Wurm 1943; Moynihan 1957; Brown et al. 1967) with only one known exception (Panov 2009). In all these postures the bill and forehead seem to be the most important signalling elements (Tinbergen and Moynihan 1952). The correlations between the time of nuptial moult and the number and strength of social preferences found in black-headed gulls bred in captivity suggest that early moulting gulls are the most attractive mates (van Rhijn and Groothuis 1987). Hence, it seems that in both sexes fully developed hood is an important part of status signalling during such interactions. The vast majority of black-headed gulls arrive at the breeding sites with head moult completed and pairing occurs mainly in the close vicinity of the breeding colony just after arrival (Glutz von Blozheim and Bauer 1982). The time of head moult completion, therefore, is similar in both sexes, and this finding is well-supported also by the results of our study.
The evolution of mutual ornamentation, such as the hood in the Black-headed Bull, is influenced by a variety of current selection pressures and such mutual ornamentation can be maintained through the selection on both sexes (Andersson 1994; Kraaijeveld et al. 2007). In the Black-headed Gull, the early nuptial moult is one of the main criteria for mate choice, which may be directly related to fitness (van Rhijn and Groothuis 1987), and both sexes prefer to pair with highly ornamented partners (Indykiewicz et al. 2017). As the hood and black wingtip in this species may have a signal function in both sexes, then at the time of mating, birds should have the best possible developed dark hood to gain a “good” mate and increase the chance of producing offspring. Unmated males of the Black-headed Gull advertise randomly, whereas unmated females direct mating calls to specific individuals rather than to a large group of potential mates and thus are far more demanding in the way they acquire a mate than males (van Rhijn and Groothuis 1987). Earlier moult is often correlated with the condition of an individual (Pap et al. 2008; Danner et al. 2015; Machín et al. 2018), which was also found in the Black-headed Gull (van Rhijn and Groothuis 1987). Moreover, in the bBack-headed Gull the earlier the moult of head proceeds, the higher the number of eggs laid by females or eggs sired by males (van Rhijn and Groothuis 1987). Hence, it seems that early moulting individuals have higher fitness than individuals which moult later in the season, and it may be assumed that males that start moulting earlier are more attractive to females. However, in gulls and terns, males are more active than females in social competition over resources other than mates and they are the more aggressive sex (Pierotti 1981; Southern 1981; Gwiazda and Ledwoń 2015). Females being smaller than males are more inclined to dominance by males when foraging in flocks in winter. Indeed, in our study, we used food bait to attract gulls to the loop trap and females composed only 27% of captured birds. It seems plausible that females as less competitive may be chased away from food resources in the non-breeding season and this may result in better body condition of males and their earlier onset of pre-breeding moult.
Data availability
The data are available upon reasonable request to the corresponding author.
Change history
14 August 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10336-023-02095-3
References
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Bairlein F, Dierschke J, Dierschke V, Salewski V, Geiter O, Hüppop K, Kӧppen U, Fiedler W (2014) Atlas des Vogelzugs. AULA-Verlag, Wiesbaden
Beltran RS, Burns JM, Breed GA (2018) Convergence of biannual moulting strategies across birds and mammals. Proc R Soc B 285:20180318. https://doi.org/10.1098/rspb.2018.0318
Bókony V, Garamszegi LZ, Hirschenhauser K, Liker A (2008) Testosterone and melanin-based black plumage coloration: a comparative study. Behav Ecol Sociobiol 62:1229–1238. https://doi.org/10.1007/s00265-008-0551-2
Bønløkke J, Madsen JJ, Thorup K, Pedersen KT, Bjerrum M, Rahbek C (2006) Dansk Trækfugleatlas. Rhodos, Humlebæk
Brown RGB, Blurton Jones NG, Hussell DJT (1967) The breeding behaviour of Sabine’s Gull, Xema sabini. Behaviour 28:110–140. https://doi.org/10.1163/156853967x00208
Burnham KP, Anderson DR (2002) Model Selection and Multimodel inference: a practical information-theoretic approach. Springer, New York
Busse P, Meissner W (2015) Bird ringing station manual. De Gruyter Open Ltd, Warsaw. https://doi.org/10.2478/9788376560533
Butcher GS, Rohwer S (1989) The evolution of conspicuous and distinctive coloration for communication in birds. In: Power DM (ed) Current ornithology. Plenum Press, New York, pp 51–97
Cepák J, Klvaňa P, Formánek J, Horák D, Jelínek M, Schrӧpfer L, Škopek J, Zárybnický J (2008) Atlas migrace ptáků Českéa Slovenské republiky. Aventinum, Praha
Černy W (1940) Durchuzg der Lachmöwe in Prag, nebst Bemerkungen über die Kopfmauser. Mitt Ver Sächs Orn 6:109–116
Cleere N (2002) Aberrant plumage in the Tawny Frogmouth, Podargus strigoides. Emu 102:195. https://doi.org/10.1071/mu01015
Crary AL, Rodewald PG (2012) Plumage coloration and ornamentation as predictors of nest survival and number of young fledged in Yellow Warblers. J Field Ornithol 83:130–140. https://doi.org/10.1111/j.1557-9263.2012.00363.x
Danner RM, Greenberg RS, Danner JE, Walters JR (2015) Winter food limits timing of prealternate moult in a short-distance migratory bird. Funct Ecol 29:259–267. https://doi.org/10.1111/1365-2435.12322
Erni B, Bonnevie BT, Oschadleus H-D, Altwegg R, Underhill LG (2013) moult: an R package to analyze moulting birds. J Stat Soft 52:1–23. https://doi.org/10.18637/jss.v052.i08
Estep LK, Shawkey MD, Hill GE (2006) Carotenoid-based breast plumage colour, body condition and clutch size in red fodies (Foudia madagascariensis). Ostrich 77:164–169. https://doi.org/10.2989/00306520609485528
Fijn RC, Govers LL, Lutterop D, Middelveld RP, van Bemmelen RSA (2022) Evidence of nocturnal migration over sea and sex-specific migration distance of Dutch Black-headed Gulls. Ardea 110:15–29. https://doi.org/10.5253/arde.v110i1.a8
Franck D, Epprecht W (1959) Zur Kopfgefiedermauser der Lachmöwe (Larus ridibundus L.) im Frühjahr. Ornithol Beobachter 56:101–109
Fransson T, Ӧsterblom H, Hall-Karlsson S (2008) Svensk ringmӓrkningsatlas, vol 2. Naturhistoriska riksmuseet, Stockholm
Giunchi D, Caccamo C, Pollonara E (2008) Pattern of wing moult and its relationship to breeding in the Eurasian Stone-curlew Burhinus oedicnemus. Ardea 96:251–260. https://doi.org/10.5253/078.096.0210
Gloe P (1983) Unter welchen Bedingungen beginnen Lachmöwen (Larus ridibundus) mit der Mauser des Kopfgefieders? Orn Mitt 35:283–287
Gloe P (1984) Möwen-Rastbestände im Hafen von Büsum (Westküste Schleswig-Holsteins). Seevögel 5:37–39
Glutz von Blozheim UN, Bauer KM (1982) Handbuch der Vögel Mitteleuropas. 8/I. Akademische Verlagsgesellschaft, Wiesbaden
Grant PJ (1986) Gulls: a guide to identification. T and AD Poyser, London
Gwiazda R, Ledwoń M (2015) Sex-specific foraging behaviour of the Whiskered Tern (Chlidonias hybrida) during the breeding season. Ornis Fenn 92:15–22
Hawkins GL, Hill GE, Mercadante A (2012) Delayed plumage maturation and delayed reproductive investment in birds. Biol Rev 87:257–274. https://doi.org/10.1111/j.1469-185x.2011.00193.x
Hill GE (1991) Plumage coloration is a sexually-selected indicator of male quality. Nature 350:337–339. https://doi.org/10.1038/350337a0
Hill GE (2006) Female mate choice for ornamental coloration. In: Hill GE, McGraw KJ (eds) Bird coloration II. Function and evolution. Harvard University Press, Cambridge, pp 137–200
Indykiewicz P, Podlaszczuk P, Surmacki A, Kudelska K, Kosicki J, Kamiński M, Minias P (2017) Scale-of-choice effect in the assortative mating by multiple ornamental and non-ornamental characters in the black-headed gull. Behav Ecol Sociobiol 71:183. https://doi.org/10.1007/s00265-017-2411-4
Karlionova N, Meissner W, Pinchuk P (2008) Differential development of breeding plumage in adult and second-year male Ruffs Philomachus pugnax. Ardea 96:39–45. https://doi.org/10.5253/078.096.0105
Kraaijeveld K, Kraaijeveld-Smit FJL, Komdeur J (2007) The evolution of mutual ornamentation. Anim Behav 74:657–677. https://doi.org/10.1016/j.anbehav.2006.12.027
Kralj J, Barišić S, Tutiš V, Ćiković D (2013) Atlas selidbe ptica Hrvatske. Hrvatska Akademija Znanosti i Umjetnosti, Zagreb
Landman A, Thaler ME (1984) Zum Vorkommen und Status der Lachmöwe (Larus ridibundus) in Nordtirol (Aves: Laridae). Ber Nat-Med Verein Innsbruck 71:187–198
Machín P, Remisiewicz M, Fernández-Elipe J, Jukema J, Klaassen RHG (2018) Conditions at the breeding grounds and migration strategy shape different moult patterns of two populations of Eurasian golden plover Pluvialis apricaria. J Avian Biol. https://doi.org/10.1111/jav.01709
MacKinnon GE, Coulson JC (1987) The temporal and geographical distribution of Continental Black-headed Gulls Larus ridibundus in the British Isles. Bird Study 34:1–9. https://doi.org/10.1080/00063658709476927
Malling Olsen K (2018) Gulls of the world. A photographic guide. Christopher Helm, London
Mazur A, Remisiewicz M, Underhill LG (2021) Sex-specific patterns of fuelling and pre-breeding body moult of Little Stints Calidris minuta in South Africa. Ibis 163:99–112. https://doi.org/10.1111/ibi.12840
Meissner W (2003) Spring passage of gulls near Rozewie Cap. Not Orn 44:179–186 (In Polish with English summary)
Meissner W, Fischer I (2017) Sexing of common gull, Larus canus, using linear measurements. Folia Zool 66:183–188. https://doi.org/10.25225/fozo.v66.i3.a6.2017
Meissner W, Remisiewicz M, Gogga P (2012) Sex and age differences in the development of breeding plumage in the Wood Sandpiper Tringa glareola during spring migration in north-eastern Poland. Ornis Fenn 89:44–52
Minias P, Indykiewicz P, Nowakowski JJ, Ledwoń M, Kowalski J, Betleja J, Dulisz B, Chyb A, Janiszewski T (2019) Melanin-based plumage ornamentation signals condition and physiological stress in the Black-headed Gull. J Ornithol 160:1159–1169. https://doi.org/10.1007/s10336-019-01690-7
Moynihan M (1957) Some aspects of reproductive behavior in the Black-Headed Gull (Larus ridibundus L.) and related species. Behav Suppl 4:1–201. https://doi.org/10.1086/402159
Mundy NI (2006) Genetic basis of color variation in wild birds. In: Hill GE, McGraw KJ (eds) Bird coloration, vol 1. Harvard University Press, Cambridge, pp 469–506
Newton I (2009) Moult and plumage. Ringing Migr 24:220–226. https://doi.org/10.1080/03078698.2009.9674395
Noble GK, Wurm M (1943) The social behavior of the Laughing Gull. Ann N Y Acad Sci 45:179–220. https://doi.org/10.1111/j.1749-6632.1943.tb47952.x
Oring LW, Reed JM, Colwell MA, Lank DB, Maxson SJ (1991) Factors regulating mating success and reproductive success in spotted sandpipers (Actitis macularia). Behav Ecol Sociobiol 28:433–442. https://doi.org/10.1007/bf00164125
Oschadleus DH, Underhill LG (2008) Primary moult of adult Red-billed Queleas (Quelea quelea) in southern Africa in relation to patterns of movement. Emu 108:331–339. https://doi.org/10.1071/mu08019
Palomares LE, Arroyo BE, Marchamalo J, Sainz JJ, Voslamber B (1997) Sex- and age-related biometric variation of Black-headed Gulls Larus ridibundus in Western European populations. Bird Study 44:310–317. https://doi.org/10.1080/00063659709461066
Panov EN (2009) The social and communication behaviour of the Great Black-headed Gull. British Birds 102:72–83
Pap PL, Vágási CI, Czirják GÁ, Barta Z (2008) Diet quality affects postnuptial molting and feather quality of the house sparrow (Passer domesticus): interaction with humoral immune function? Can J Zool 86:834–842. https://doi.org/10.1139/z08-060
Paterson AM (1993) Development of head moult of Black-headed Gulls Larus ridibundus in southern Spain. Seabirds 15:68–71
Pierotti R (1981) Male and female parental roles in the Western Gull under different environmental conditions. Auk 98:532–549
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Remisiewicz M, Tree AJ, Underhill LG, Taylor PB (2010) Rapid or slow moult? The choice of a primary moult strategy by immature wood sandpipers Tringa glareola in southern Africa. J Ornithol 151:429–441. https://doi.org/10.1007/s10336-009-0473-4
Rimmer CC, Tietz JR (2001) An adult male Blackpoll Warbler in female-like plumage. J Field Ornithol 72:365–368. https://doi.org/10.1648/0273-8570-72.3.365
Ryder JP (1975) Egg-laying, egg size, and success in relation to immature-mature plumage of Ring-billed Gulls. Wilson Bull 75:534–542
Schwarzenbach FH (1960) Zur Kopfgefiedermauser der Lachmöwe (Larus ridibundus L.) im Frühjahr. Ornithol Beobachter 57:177–186
Serra L, Clark NA, Clark JA (2006) Primary moult, body mass and migration of Grey Plovers Pluvialis squatarola in Britain. Ibis 148:292–301
Southern LK (1981) Sex-related differences in territorial aggression by Ring-billed Gulls. Auk 98:179–181
Spaans AL (2000) Grote plaatstrouw van Kokmeeuwen Larus ridibundus aan een winterkwartier in Den Haag. Limosa 73:87–96
Szinai P (1998) Recoveries of Black-headed Gulls (Larus ridibundus L.) from Rétszilas, Hungary. Ornis Hungarica 8, suppl. 1:199–203
Tinbergen N, Moynihan M (1952) Head-flagging in the Black-headed Gull; its function and origin. British Birds 45:19–22
Underhill LG, Zucchini W (1988) A model for avian primary moult. Ibis 130:358–372
Underhill LG, Zucchini W, Summers RW (1990) A model for avian primary moult-data types based on migration strategies and an example using the Redshank Tringa totanus. Ibis 132:118–123
Valkama J, Saurola P, Lehikoinen A, Lehikoinen E, Piha M, Sola P, Velmala W (2014) Suomen Rengastusatlas, vol II. Finnish Museum of Natural History and Ministry of Environment, Helsinki
van der Jeugd HP, Blaakmeer KB (2001) Teenage love: the importance of trial liaisons, subadult plumage and early pairing in barnacle geese. Anim Behav 62:1075–1083
van Grouw H (2017) The dark side of birds: melanism—facts and fiction. Bull British Ornithol Club 137:12–36
van Dijk K, Majoor F (1995) Aflezen van metalen ringen bij Kokmeeuwen in Groningen en het Gooi. Vogeljaar 43:145–154
van Rhijn J, Groothuis T (1987) On the mechanism of mate selection in Black-headed Gulls. Behaviour 100:134–169. https://doi.org/10.1163/156853987x00116
Wernham C, Toms M, Marchant J, Clark J, Siriwardena G, Baillie S (2002) The migration atlas: movements of the birds of Britain and Ireland. T and AD Poyser, London
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-Verlag New, York
Wiebe KL, Vitousek MN (2015) Melanin plumage ornaments in both sexes of Northern Flicker are associated with body condition and predict reproductive output independent of age. Auk 132:507–517. https://doi.org/10.1642/auk-14-281.1
Yezerinac SM, Weatherhead PJ (1997) Extra-pair mating, male plumage coloration and sexual selection in Yellow Warblers (Dendroica petechia). Proc Royal Soc B 264:527–532. https://doi.org/10.1098/rspb.1997.0075
Acknowledgements
We are grateful to all colleagues who have helped in the fieldwork and particularly to the ringers of Waterbird Research Group KULING, especially to Szymon Bzoma, Mateusz Ściborski, and Cezary Wójcik, who collected a significant amount of data on birds during ringing. This is Waterbird Research Group KULING Contribution No. 175. The field study complies with the current laws of Poland.
Funding
This research was supported by the University of Gdańsk and Waterbird Research Group KULING and did not receive any specific grant from funding agencies in the public or commercial sectors.
Author information
Authors and Affiliations
Contributions
WM developed the ideas, designed the study, carried out the fieldwork and analysed the data, EC carried out the fieldwork and analysed the data, AO supervised data collection in 2020 and preliminary analyses, WM and AO took a lead in writing, review, and editing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare there is no conflict of interest.
Additional information
Communicated by C. Barbraud.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Meissner, W., Czaplewska, E. & Ożarowska, A. Sex- and age-dependent breeding plumage acquisition in monomorphic species, the Black-headed Gull Chroicocephalus ridibundus. J Ornithol 165, 81–89 (2024). https://doi.org/10.1007/s10336-023-02089-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-023-02089-1