Abstract
The Short-eared Owl (Asio flammeus) inhabits open grasslands and belongs to the ground-breeding birds that have experienced the most severe declines during recent decades. Here, we studied nest and fledgling survival of the owl species in relation to (i) habitat composition, (ii) vegetation structure, (iii) weather conditions and (iv) vole abundance. The study was conducted on the East Frisian Island of Spiekeroog (southern North Sea, Lower Saxony, Germany), which harbours one of the last remaining permanent populations of the species in Central Europe. With a mean hatching success of 5.6 young per nest (N = 34) and an average probability of nest survival of 0.9 (N = 28), values ascertained in this study exceeded those reported in previous research. We attribute this to the special environmental conditions on the island, i.e. (i) the absence of mammalian mesopredators such as the Red Fox (Vulpes vulpes), (ii) nearly no disturbance through agricultural measures and (iii) low level of human disturbance due to legal regulations of the National Park. By contrast, the survival of fledglings was lower than survival of nests and varied considerably between the investigated years. Weather conditions were the key driver of fledgling survival. Maximum wind speed and sunshine duration had a negative effect on the probability that chicks successfully fledged. Both lead to reduced hunting success, and the former is also associated with increased costs for thermoregulation. Consequently, increasing frequency of extreme weather events caused by climate change could negatively impact the breeding success of vole-dependent raptors, such as the Short-eared Owl.
Zusammenfassung
Wetterbedingungen bestimmen den Fortpflanzungserfolg einer bodenbrütenden Eulenart in natürlichen Küstendünen.
Die Sumpfohreule (Asio flammeus) besiedelt offenes Grünland und zählt zu den bodenbrütenden Vögeln, bei denen in den letzten Jahrzehnten die stärksten Bestandsrückgänge zu verzeichnen waren. Wir untersuchten die Überlebenswahrscheinlichkeit von Nestern und Jungvögeln der Sumpfohreule in Abhängigkeit von (i) Habitatzusammensetzung, (ii) Vegetationsstruktur, (iii) Wetterbedingungen und (iv) Wühlmausvorkommen. Die Studie wurde auf der Ostfriesischen Insel Spiekeroog durchgeführt, die eine der letzten verbleibenden dauerhaften Population der Art in Mitteleuropa beherbergt. Mit einem durchschnittlichen Schlupferfolg von 5.6 Jungtieren pro Nest (N = 34) und einer durchschnittlichen Überlebenswahrscheinlichkeit der Nester von 0.9 (N = 28) war der festgestellte Nesterfolg erheblich größer als in bisherigen Studien festgestellt. Wir führen dies auf die besonderen Umweltbedingungen auf der Insel und insbesondere auf (i) das Fehlen von Mesoprädatoren wie dem Rotfuchs (Vulpes vulpes), (ii) das fast vollständige Fehlen von Störungen durch landwirtschaftliche Maßnahmen und (iii) eine geringe Störungsintensität durch die gesetzlichen Bestimmungen des Nationalpark zurück. Die Überlebensrate der Jungvögel war dagegen deutlich geringer und variierte erheblich zwischen den Untersuchungsjahren. Wetterbedingungen hatten den größten Effekt auf das Überleben der Jungvögel. Eine hohe Sonnenscheindauer und insbesondere hohe Windgeschwindigkeiten wirkten sich stark negativ auf die Überlebenswahrscheinlichkeit junger Sumpfohreulen aus. Beides führt zu einem verminderten Jagderfolg und somit zu einer erschwerten Nahrungsversorgung der Jungvögel. Hohe Windgeschwindigkeiten führen gleichzeitig zu erhöhten Kosten für die Thermoregulation und einem damit verbundenen höheren Nahrungsbedarf. Folglich könnte sich eine zunehmende Häufigkeit von extremen Wetterereignissen im Zuge des Klimawandels negativ auf den Bruterfolg von auf Wühlmäuse spezialisierten Greifvögeln, wie der Sumpfohreule, auswirken.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Throughout Europe, agricultural intensification has led to a dramatic decline in biodiversity (Donald et al. 2006; Emmerson et al. 2016; Reif and Hanzelka 2020). Consequently, birds of open grasslands are among the most rapidly declining bird species. The loss is particularly severe in ground-nesting species (Van Turnhout et al. 2010; Kamp et al. 2021). Habitat loss due to agricultural intensification, an increase in nest disturbance through agricultural activities during the breeding season and higher predation rates because of growing populations of mesopredators, such as the Red Fox (Vulpes vulpes), are considered to be the main drivers of the sharp decline in ground-nesting birds (Newton 2017; Roos et al. 2018).
The Short-eared Owl (Asio flammeus) inhabits open grasslands and belongs to the ground-breeding birds that have experienced the most severe declines during recent decades (BirdLife International 2004). As a result, it is considered a species of conservation concern in Europe (Calladine et al. 2012; Fernandez-Bellon et al. 2020) and threatened with extinction in Germany (Ryslavy et al. 2020). The owl has especially suffered from habitat loss and degradation due to agricultural intensification, increased predation and reduced prey availability (Fernández-Bellon et al. 2020).
Nest and fledgling survival crucially affect population dynamics (Ludwig et al. 2018). Therefore, the identification of the key drivers of reproductive success is decisive for the conservation of threatened species (Green 1999; Bro et al. 2000). This is especially true for species with strong population fluctuations (Nuijten et al. 2020). Populations of the Short-eared Owl are known to heavily oscillate depending on local vole populations (Korpimäi and Norrdahl 1991). However, overall, our knowledge on the environmental parameters that determine reproductive success in this species is scarce (Holt 1992; Fernandez-Bellon et al. 2020). Additionally, habitats of the Short-eared Owl are predicted to become increasingly degraded in some areas in the course of ongoing climate change (Miller et al. 2020). Therefore, there is an urgent need to identify the key drivers of nest and fledgling survival as a basis for the development of effective conservation strategies in times of global change (Holt 1992; Fernandez-Bellon et al. 2020).
Here, we studied nest and fledgling survival of the Short-eared Owl in relation to (i) habitat composition, (ii) vegetation structure, (iii) weather conditions and (iv) vole abundance. The study was conducted on the East Frisian Island of Spiekeroog (southern North Sea, Lower Saxony, Germany), which harbours one of the last remaining consistently occupied populations of the Short-eared Owl in Central Europe. The study area is part of the Wadden Sea National Park of Lower Saxony and the Wadden Sea World Heritage site. It is characterized by natural dunes and marshes free from predatory mammals and largely undisturbed by agricultural activities or humans. The aim of this study was to assess the relative influence of weather and habitat on reproductive success in a breeding population of the Short-eared Owl that does not show the typical strong fluctuations associated with vole cycles.
Materials and methods
Study species
The nominate subspecies of the Short-eared Owl (Asio flammeus flammeus) has a large Holarctic breeding range (Keller et al. 2020). However, across its distribution area, abundance of the species varies strongly. In Central Europe, characterized by intensive agriculture (Ellenberg and Leuschner 2010), the species is very rare (Keller et al. 2020) and greater breeding densities are strongly dependent on a high local abundance of Common vole (Microtus arvalis) (Gedeon et al. 2014). Within Central Europe, both natural (e.g., bogs, coastal dunes, and marshes) and man-made habitats (e.g., agricultural land) are used for breeding (Keller et al. 2020). Habitat loss and degradation, increased predation and reduced prey availability are considered to be the main threats of the Short-eared Owl in this part of its range (Fernández-Bellon et al. 2020).
The Short-eared Owl is a ground-breeding species. In northern and central Europe nesting usually occurs from March to May, and clutch size ranges from 2 to 8. Eggs are mostly laid at daily intervals, and incubation of eggs takes an average of 27 days. Fledglings leave the nest and hide in the surrounding vegetation at an age of 12–17 days. Their parents continue to feed them until they fledge at the age of 24–27 days (Hardey et al. 2013). On the East Frisian Islands, dune grasslands, dune heath, salt marshes and salty dunes are the main foraging habitats of the species (Kämpfer et al. 2020). For further information about the habitat types see Petersen et al. (2014).
In Germany, population size is estimated to be 50–180 territories (2005–2009) (Gedeon et al. 2014). In years with vole outbreaks, the number of territories can strongly increase (Krüger 2019). For example, in 2019, more than 200 breeding pairs were detected, mostly nesting in improved grasslands. However, in most years, population size is at the lower edge of the population estimate. Within Germany, occurrence of the Short-eared Owl is mainly restricted to the North-Sea coasts and the Wadden Sea Islands (Gedeon et al. 2014; Kämpfer and Fartmann 2020). The only permanent German breeding population with an average of 36 territories between 1993 and 2018 was located on the East Frisian Islands (Kämpfer and Fartmann 2020). Here, the island of Spiekeroog was the abundance hotspot, with 10–15 breeding pairs per year. Even in years with low vole abundance, nest camera data revealed that voles are by far the most important food for chicks on this island (Klock 2018).
Study area
The study was conducted on the East Frisian Island of Spiekeroog (southern North Sea, Lower Saxony, Germany). Spiekeroog is about 2 km wide and 10 km long, resulting in a total area of 18 km2 (Petersen and Pott 2005). An Atlantic climate with a mean annual temperature of 9.6 °C and a mean precipitation of 752 mm characterise the study area (weather station: Norderney; long-term mean: 1981–2010) (Deutscher Wetterdienst, 2019). The East Frisian Islands are sandy barrier islands and are influenced by tides. The main habitats on the islands are beaches (18%), natural dune grasslands (13%), mudflats (13%), marshes (35%), built-up areas (4%), and dune heath (4%). Further habitats that cover smaller areas are copses (3%), white dunes (2%), shrubberies (2%), dune slacks (1%), reeds (1%), semi natural grassland and transition zones between marshes and natural dune grasslands called salty dune (1%) (Petersen and Pott 2005; Petersen et al. 2014). The study area is part of the Wadden Sea National Park of Lower Saxony and the Wadden Sea World Heritage site. During the breeding season, access is prohibited for humans in most areas, except in small parts in the so called 'recreational zone' or on designated roads and paths. Dogs must generally be on a leash. Due to intensive public relations work, visitor management and the use of National Park rangers and volunteers to control entry bans, protected areas are rarely disturbed. Only small parts of the island, primarily salt marshes, are grazed by livestock. The study area is free of mammalian predators except for domestic cats (Felis catus) (Walter and Kleinkuhle, 2008), common rats (Rattus norvegicus) and hedgehogs (Erinaceus europaeus) (Andretzke and Oltmanns 2016).
Sampling design
Nest detection, nest, and fledgling survival
Within the study area, we searched for nests of the Short-eared Owl from March to July in 2011, 2015 and 2017–2019. For the identification of nest sites, we used key behaviours that were indicative of territoriality, such as carrying prey to a potential nest, giving alarm calls, mobbing potential predators, courtship display (wing clapping) and constant perching in the open during daylight periods (indicating a male near an incubating female) (Calladine et al. 2010). During incubation of an average of 27 days (Hardey et al. 2013), all nests found before hatching (n = 28, 511 exposure days) were monitored at 6–9-day intervals to document clutch size, number of hatched chicks and general nest fate, until all fledglings left the nest.
All chicks were banded with an aluminium ring (Ornithological station “Vogelwarte Helgoland”) between May and July, right before they left the nest. To improve rediscovery rates of fledglings in their hiding places and to determine their individual fate more precisely, we additionally used coded radio-transmitters (Biotrack ACT-626, 1.3 g), a hand-held antenna (Lotek LiteFlex VHF Yagi) and a receiver (Lotek SRX800). In 2018 and 2019, we radio-tagged 15 fledglings in both years. Radio-tags measured 1.5 × 0.7 cm and were glued on linen fabric of 2.3 × 1.5 cm using universal adhesive (UHU Super Strong) to increase surface area. Subsequently, the radio tags were glued on a feather-free spot on the back of fledglings by applying surgical cement (Perma-Type), which is free from skin-irritating substances and remains flexible even after fast drying. Including material for attachment, tags weighed 2.2 g, while tagged birds weighed 207–386 g (mean 281 g). Thus, mass of radio tags was at most 1.1% of a bird’s body mass and, hence, below the upper recommended load limit of 5% (Kenward 2001). Rediscovered ringed and radio-tagged fledglings are hereinafter termed tagged fledglings. All tagged birds were checked every 5–7 days until their flight ability was large enough to flee from approaching humans and radio tags fell off due to higher mobility of the birds. In this case, fledglings were considered successfully fledged. The geographic position of the tagged fledglings was recorded using a GPS-device. Altogether, 38 tagged fledglings could be included in the analysis of fledgling survival, representing a total of 769 exposure days.
Daily survival rate (DSR) of a nest was defined as the probability that at least one egg within a nest survived a single day (Dinsmore et al. 2002). By contrast, DSR of fledglings considered the survival of each fledgling during a period of 24 h. The probability of nest survival during the nesting period and fledglings' survival during the fledgling period (in both cases 27 days, Hardey et al. 2013), were calculated as the product of 27 consecutive daily nest-survival rates of nests/fledglings (DSR^27) (cf. Dinsmore et al. 2002). Nests for which nest fate or laying date could not be determined unequivocally or those which were found after some of the chicks had already left the nest were excluded from further analysis, resulting in different sample sizes for different parameters (Table 1).
Vole abundances
Vole abundance was sampled in 2018 and 2019 within the four main foraging habitats of the Short-eared Owl in the study area: dune heath, dune grassland, salt marsh and salty dune (see ‘study area’) (Hirschberg 2018; Kämpfer et al. 2020). We randomly selected 12 plots (three per habitat type) using the function ‘create random points’ in ArcGIS 10.4. For trapping of voles, we arranged 25 Longworth traps in a square grid of 5 × 5 traps, each separated by 10 m (cf. Jareño et al. 2014). Trap deployment was guided by traces, corridors and burrows in an effort to increase probability of catch (Gurnell and Flowerdew 2006). The traps were equipped with an apple (as a water substitute), oats and wheat and were insulated with wood chips to protect the voles from hypothermia (Jareño et al. 2014). Voles were individually marked by fur cutting according to Gurnell and Flowerdew (2006). Trapping was conducted at the end of June, when most of the breeding owls were rearing young and the need for food was particularly high. The traps were opened 8 h after placing them in the plot and from then on left open for 24 h. During this period, traps were checked every 8 h. The number of captures per plot was converted to the number of captures per 100 trap nights (recaptured individuals were not included) (cf. Steen and Gibbs 2004).
Environmental parameters
To determine the environmental drivers of nest and fledgling survival, we used data on (i) habitat type, (ii) vegetation structure and (iii) weather. We analysed habitat composition within the home range of tagged fledglings. Home range was determined by applying the minimum-convex-polygon (MCP) method (White and Garrot 1990) using the function ‘minimum bounding geometry’ in ArcGIS 10.4. The resulting polygons were intersected with habitat data available through the Trilateral Monitoring and Assessment Program (TMAP) (Wadden Sea National Park of Lower Saxony 2017). The proportion of each habitat type within the home range was then calculated for every tagged bird. Furthermore, we measured mean vegetation height (accuracy: 1 cm) using a ruler and estimated the percentage cover of bare ground, herb layer, mosses, shrubs and litter in an area of 10 m × 10 m around each nest in June. Weather data comprised maximum wind speed (m/s), mean wind speed (m/s), precipitation sum, sunshine duration, temperature, and relative humidity per day (weather station island of Norderney, 20 km west of the study area, German Meteorological Service (DWD 2020)).
To test the effects of habitat composition and vegetation structure on the number of successfully fledged young per nest, we also applied the methods described above for the tagged fledglings for each individual nest. However, the assessment of the home range was based on the location of all fledglings per nest and not only a single bird.
To assess the impact of habitat structure on vole abundance, we measured vegetation height (accuracy: 1 cm) as well as the percentage cover of bare ground, herb layer, mosses, shrubs and litter in three subplots of 3 m × 3 m size within each of the 12 plots in which small mammal trapping was conducted in June 2018 (see ‘sampling design’). For further analysis, we used the mean of the three subplots.
Statistical analysis
All statistical analyses were conducted using the software R 4.0.3. (R Development Core Team 2021). To account for undetected nests in survival estimates and to incorporate explanatory variables that may explain variation in nest and fledgling survival, daily survival rates (DSR) were modelled by applying nest-survival models (NSMs) in the programme ‘MARK’ (Dinsmore and Dinsmore 2007; Cooch and White 2019). Analysis was performed using the R-interface ‘RMark’ version 2.2.7 (Laake 2020). Since for the evaluation of nest DSR nest age (laying date) must be determined accurately (Dinsmore and Dinsmore 2007), it was calculated based on nest-monitoring, (hatching date and incomplete clutches) assuming a mean incubation period of 27 days with eggs laid at daily intervals (see ‘study species’).
In models for DSR of fledglings, we used habitat compositions of the home range as individual covariates, vegetation structures as grouped covariates and weather data as time-specific covariates (see ‘sampling design’) (Dinsmore et al. 2002). Moreover, we incorporated nest age, the number of nest siblings as well as linear and quadratic time trends and year, to account for possible annual variation, in the models (Dinsmore et al. 2002).
To avoid overfitting, firstly, four different NSMs of fledgling survival were conducted: (i) a breeding-biology model incorporating age and time dependent effects as well as number of siblings, (ii) a habitat-type model with the cover of different habitat types as predictors, (iii) a vegetation-structure model including vegetation height and cover of different vegetation layers and (iv) a weather model (Table 2). Finally, a synthesis model was generated including all significant predictors of the preceding four models.
To evaluate those variables that influence the number of successfully fledged birds per nest, we used generalized linear mixed-effects models (GLMMs) applying the ‘lme4’ package of Bates et al. (2015), with binomial error distribution and a logit link function. We used the proportion of the number of successfully fledged and died fledglings per nest as the response variable applying the function cbind (cf. Schöll and Hille 2020), and the cover of different habitat types within the home range of the fledglings of each nest and vegetation structure as predictors. Additionally, we incorporated ‘year’ as a random effect (cf. Crawley 2007).
To increase model robustness and identify the most important parameters in NSMs and GLMMs, we performed model averaging based on an information-theoretic approach (Burnham and Anderson, 2002; Grueber et al. 2011). Model averaging was performed using the ‘dredge’ function (R package MuMIn; Bartón 2019) and included only top-ranked models with ΔAICc < 2 (cf. Grueber et al. 2011). The maximum number of predictors to be included in a single model was limited to 1/10 of the sample size (Harrell et al. 1996). To avoid multi-collinearity in the models, Spearmen's rank correlations (r) were used to test for strong inter-correlations (|r|≥ 0.6) (Dormann et al. 2013). Because the cover of the herb layer and mean vegetation height were intercorrelated (r = 0.63, p < 0.001) and mean daily wind speed was intercorrelated with maximum wind speed (r = 0.78, p < 0.001), only one of the respective variables was included in the models. Based on AICc values, models including vegetation height and maximum wind speed performed better compared with models including herb layer and mean wind speed. Consequently, herb layer and mean wind speed were excluded from the analysis.
To test for differences in vole abundance between years and habitat types, we used a two-way repeated-measures ANOVA with a Bonferroni t test. The effect of vegetation structure on vole abundance was analysed using GLMM as described above, with ‘vole abundance’ as the response variable, ‘vegetation structure’ as predictors and ‘habitat type’ as a random effect.
Results
Nest and fledgling survival
Overall, in the five study years, 39 owl nests were found before the chick rearing stage (Table 1). Mean clutch size varied between 5.4 (2017) and 7.1 (2019) with an average of 6.3 eggs per nest. Probability of nest survival during the nesting period was very high and ranged between 0.82 and 1 (mean: 0.9). The egg-laying period varied from March 24th to June 15th (probably a second brood since it only contained three eggs) with April 26th as the median laying date (Table 1).
Out of 202 eggs, 178 hatched successfully (88%, Table 1), 14 eggs remained in the nest with intact surface, one chick died during hatching, three eggs were found destroyed in the surrounding of the nest and six eggs disappeared for unknown reasons. Hatching success ranged between 4.4 (2017) and 6.1 (2019) with a mean of 5.6 hatched young per nest.
Fledgling survival was much lower than nest survival and differed strongly between the two study years. In 2018, the probability of the fledglings to became fully fledged was 0.35, while in 2019 the probability was almost twice as high at 0.64.
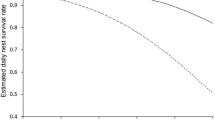
Among the five NSMs, the breeding-biology model was the only one that failed to detect significant predictors of fledgling survival (Table 2). Daily fledgling survival increased with the area of salty dunes in the home range (habitat-type model), the cover of litter around the nest (vegetation-structure model), a low daily sunshine duration (weather model) and a low daily maximum wind speed (weather model). In the synthesis model, only the two weather variables, sunshine duration and wind speed, had a significant influence on fledgling survival (Table 2, Fig. 1). By contrast, the number of successfully fledged young per nest only increased with the cover of litter within the home range of the fledglings (Table 3, Fig. 2).
Results of the synthesis NSM relationship between daily survival rate of Short-eared Owl fledglings and significant environmental parameters. Daily survival rate: probability that a fledgling survives a single day. Note the different scales in the Y-axis. For detailed results see Table 2e
Results of the GLMMs relationship between the proportion of successfully fledged young per nest and significant environmental parameters (litter cover). For detailed results see Table 3
Vole abundance
Vole abundance was almost three times higher in 2019 than in 2018 (Fig. 3). Salty dunes had the highest abundance and dune heath/grassland the lowest; salt marshes had an intermediate position. Abundance of voles in the plots increased with the cover of the litter (Table 4, Fig. 4).
Abundance (individuals per 100 trap nights) of voles (Mircrotus arvalis) in the four main foraging habitat types of the short-eared owl in 2018 and 2019. Differences were tested using two-way repeated-measures ANOVA with a Bonferroni t test. Different letters indicate significant differences between vole abundance in the habitat types (habitat types F = 8.1, P ≤ 0.01; year F = 6.3, P ≤ 0.05)
Results of the GLMM relationship between the abundance of voles (Microtus arvalis) and significant environmental parameters (litter cover). For detailed results see Table 4
Discussion
Both predation of eggs and nestlings, especially by mammalian mesopredators, are considered important drivers of reproductive failure in ground-nesting birds (Roos et al. 2018). This also applies for the Short-eared Owl (Fondell and Ball 2003). Further threats causing loss of eggs and nestlings in this owl are agricultural measures such as mowing and human disturbance (Wiggins 2004; Fernandez-Bellon et al. 2019).
In our study, hatching success and nest survival of the Short-eared Owl were extraordinarily high. With a mean hatching success of 5.6 young per nest (N = 34) and an average probability of nest survival of 0.90 (N = 28), our values from the East Frisian Island of Spiekeroog exceeded those reported in other studies (Pitelka et al. 1955; Holt 1992). We attribute this to the special environmental conditions on the island, i.e. (i) the absence of mammalian mesopredators such as the Red fox (Vulpes vulpes), (ii) nearly no disturbance through agricultural measures and (iii) a lack of human disturbance due to legal regulations of the National Park on large parts of the island.
Predatory birds are also known to cause egg and nestling loss (Roos et al. 2018). Herring gull (Larus argentatus) and Lesser black-backed gull (Larus fuscus) are numerous, and Carrion crow (Corvus corone) and Marsh harrier (Circus aeruginosus) are regular breeding birds in the study area (Gedeon et al. 2014). All four species have been identified as predators of Short-eared Owl eggs or nestlings in other study areas (Holt 1992; Wiggins 2004). We explain the virtual absence of predation through birds by the highly effective defensive behaviour of adult owls in habitats that are largely free of disturbance by humans. During field work, we regularly observed defensive behaviour, especially against carrion crows and marsh harriers but also gulls. In all observed cases the owls successfully expelled the predatory birds. By contrast, in environments where humans regularly disturb breeding owls, the nests are no longer protected by defending adults and, therefore, predation rates may increase. Consequently, we assume that the National Park concept including zoning and visitor management as well as the use of National Park rangers to control entry bans is another important tool to secure successful reproduction of the Short-eared Owl.
In contrast to hatching success and nest survival, the survival of fledglings was clearly lower and, additionally, differed between the 2 years. Weather conditions were strongly associated with fledgling's survival and were, therefore, likely a key driver. Both maximum wind speed and sunshine duration had a negative effect on the probability that chicks successfully fledged. Van Manen (2001) observed that Long-eared owls hunted most effectively at a wind speed of about 4 m/s. At higher wind velocity, the amount of prey caught decreased. Windy conditions are well known to hamper prey detection (Bradley et al. 1997) and hunting success (Fisher et al. 2004) in birds of prey. This is especially true for owls for which acoustic prey recognition plays an important role (Van Manen 2001; Kouba et al. 2017). Besides food shortage for the fledglings, periods of windy weather are also associated with increased costs for thermoregulation (Tatner 1989; Bakken et al. 2002). Both result in increased mortality of the fledglings.
The negative relationship between sunshine duration and fledgling survival can probably also be explained by a reduced hunting success (Wróbel and Bogdziewicz 2015). Foraging of Short-eared Owls is most efficient when it coincides with peaks in vole activity (Reynolds and Gorman 1999). Several studies have shown that increased cloud cover and darker conditions enhance activity of small mammals (Vickery and Bider 1981; Brown et al. 1988; Stokes et al. 2001). The authors assume this activity pattern to be an adaptation to avoid predation through predators that use visual prey detection.
Additional predictors of fledgling survival were the area of salty dunes within the home range of fledglings and the cover of litter around nests, both fostered survival rates. Salty dunes, the transition zone between high marshes and dune grasslands, usually form small-scale mosaics within high marshes and protrude them by several decimetres (Petersen and Pott 2005). Therefore, they are probably important refuges during storm surges in winter, facilitating a high abundance of voles. Indeed, salty dunes had the highest vole abundance in our study. Moreover, salty dunes surmount the surrounding high marshes and may thereby favour distant views and early predator detection.
Both the survival of fledglings and the number of successfully fledged chicks per nest increased with the cover of litter around the nest. Vole abundance was positively related to litter cover in our study. This aligns with previous findings that especially voles prefer dense herbaceous vegetation with a pronounced litter layer (Huang et al. 2010). Moreover, Short-eared Owls are known to depend on taller vegetation with high amounts of litter for nesting (Holt 1992; Swengel and Swengel 2014; Kämpfer et al. 2013). It is very likely that high amounts of litter facilitate concealment of nests and fledglings, resulting in lower predation rates (Martínez et al. 1998). Consequently, we attribute the positive effect of litter cover on fledgling survival to a (i) higher food supply due to higher prey densities in the proximity of the nests and (ii) lower predation risk due to enhanced concealment of fledglings.
Survival of fledglings was almost two times higher (0.64 vs. 0.35) and abundance of voles nearly three times higher in 2019 than in 2018. However, surprisingly, the year had no effect on survival rates in our study. Short-eared Owls are known to be highly dependent on vole abundance (Korpimäki and Norrdahl 1991; Johnson et al. 2013), resulting in unpredictable invasions in years of vole outbreaks (Kleefstra et al. 2015; Krüger 2019; Škorpíková et al. 2020). By contrast, on the East-Frisian Islands, the number of breeding pairs is relatively constant (Kämpfer and Fartmann 2020; Kämpfer et al. 2013). There are two possible explanations for the low variation in population size of Short-eared Owl on the islands: (i) the presence of sufficient alternative prey, such as birds, or (ii) relatively low fluctuations in vole abundance. Indeed, on islands in the Dutch Wadden Sea, where voles are absent, the owl diet consists of up to 90% of waders and songbirds (Schaub and Klaassen 2020). However, in our study area, even in years with lower vole abundance, such as 2018, fledglings were almost exclusively fed with voles (93%, N = 42; data of nest cameras) (Klock 2018). Accordingly, the first assumption must be rejected. By contrast, Knipping et al. (2020) did not observe any cyclic variation in vole abundance on the East-Frisian Islands over a period of 6 years, which points to the second explanation. Accordingly, for the Short-eared Owl, the availability of voles seems to be sufficient even in years with relatively low abundance, if weather and habitat conditions are favourable (see above). However, further research is necessary and should include long-term monitoring of vole populations as well as the effects of storm surges on vole abundance.
In conclusion, our study highlighted the prime importance of natural barrier islands largely free of human disturbance and mammalian mesopredators for the survival of a threatened ground-breeding bird of prey. So far, the availability of voles was considered the main predictor of reproductive success in the Short-eared Owl (Korpimäki and Norrdahl 1991; Johnson et al. 2013). Our study, however, now revealed that extreme weather events, here periods of strong wind, were the key driver of reproductive failure. Consequently, climate change might threaten vole-dependent raptors, such as the Short-eared Owl, not only by alterations in temperature and precipitation (Miller et al. 2020), habitat degradation (Miller et al. 2020) or trophic interactions like dampening of vole cycles (Millon et al. 2014) but also through extreme weather events, which are predicted to increase due to climate change (IPCC 2021). To assess the potential impacts of climate change on birds of conservation concern more precisely and to develop suitable adaptation strategies, further research on the effects of extreme weather events is urgently needed.
References
Andretzke H, Oltmanns B (2016) What really helps breeding birds? presentation and evaluation of protective measures in the National Park “Niedersächsisches Wattenmeer”, exemplified by the East-Frisian island of Norderney. Vogelkundl Ber Niedersachs 44:195–215 (German with English abstract)
Bakken GS, Williams JB, Ricklefs RE (2002) Metabolic response to wind of downy chicks of arctic-breeding shorebirds (Scolopacidae). J Exp Biol 205:3435–3443. https://doi.org/10.1242/jeb.205.22.3435
Bartón K (2019) MuMIn: multi-model inference: R package
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw. https://doi.org/10.18637/jss.v067.i01
BirdLife International (2004) Birds in Europe: population estimates, trends and conservation status. BirdLife International, Cambridge
Bradley M, Johnstone R, Court G, Duncan T (1997) Influence of weather on breeding success of peregrine falcons in the arctic. Auk 114:786–791
Bro E, Sarrazin F, Clobert J, Reitz F (2000) Demography and the decline of the grey partridge Perdix perdix in France. J Appl Ecol 37:432–448
Brown JS, Kotler BP, Smith RJ, Wirtz WO (1988) The effects of owl predation on the foraging behaviour of heteromyid rodents. Oecologia 76:408–415. https://doi.org/10.1007/BF00377036
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Calladine J, Garner G, Wernham C, Buxton N (2010) Variation in the diurnal activity of breeding short-eared owls Asio flammeus: implications for their survey and monitoring. Bird Study 57:89–99. https://doi.org/10.1080/00063650903437503
Calladine J, du Feu C, du Feu R (2012) Changing migration patterns of the Short-eared Owl Asio flammeus in Europe: an analysis of ringing recoveries. J Ornithol 153:691–698. https://doi.org/10.1007/s10336-011-0786-y
Cooch EG, White GC (Eds) (2019) Program MARK. A gentle introduction, 19th edn http://www.phidot.org/software/mark/docs/book. Accessed 08 November 2020
Crawley MJ (2007) The R Book. Wiley, Chichester
Dinsmore SJ, Dinsmore JJ (2007) Modeling avian nest survival in program MARK. Stud Avian Biol 34:73–83
Dinsmore S, White GC, Knopf FL (2002) Advanced techniques for modeling avian nest survival. Ecology 83:3476–3488. https://doi.org/10.1890/0012-9658(2002)083[3476:ATFMAN]2.0.CO;2
Donald PF, Sanderson FJ, Burfield IJ, Van Bommel IJ (2006) Further evidence of continent-wide impacts of agricultural intensification on European farmland birds, 1990–2000. Agric Ecosyst Environ 116:189–196. https://doi.org/10.1016/j.agee.2006.02.007
Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carré G, Marquéz JRG, Gruber B, Lafourcade B, Leitão PJ, Münkemüller T, McClean C, Osborne PE, Reineking B, Schröder B, Skidmore AK, Zurell D, Lautenbach S (2013) Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36:27–46. https://doi.org/10.1111/j.1600-0587.2012.07348.x
DWD (Deutscher Wetterdienst) (2020) Climate data Germany—monthly and daily values. https://www.dwd.de/DE/leistungen/klimadatendeutschland/klarchivtagmonat.html?nn=16102. Accessed 08 August 2020
Ellenberg H, Leuschner C (2010) Vegetation mitteleuropas mit den alpen, 6th edn. Eugen Ulmer, Stuttgart
Emmerson M, Morales MB, Oñate JJ, Batáry P, Berendse F, Liira J, Aavik T, Guerrero I, Bommarco R, Eggers S, Pärt T, Tscharntke T, Weisser W, Clement L, Bengtsson J (2016) How agricultural intensification affects biodiversity and ecosystem services. Large-Scale Ecol 55:43–97
Fernández-Bellon D, Lusby J, Bos J, Schaub T, McCarthy A, Caravaggi A, Irwin S, O’Halloran J (2020) Expert knowledge assessment of threats and conservation strategies for breeding Hen Harrier and Short-eared Owl across Europe. Bird Conserv Int. https://doi.org/10.1017/S0959270920000349
Fisher RJ, Poulin RG, Todd LD, Brigham RM (2004) Nest stage, wind speed, and air temperature affect the nest defence behaviours of burrowing owls. Can J Zool 82:707–713. https://doi.org/10.1139/z04-035
Fondell TF, Ball IJ (2004) Density and success of bird nests relative to grazing on western Montana grasslands. Bio Conserv 117:203–213. https://doi.org/10.1016/S0006-3207(03)00293-3
Gedeon K, Grüneberg C Mitschke A, Sudfeldt C (eds) (2014) Atlas Deutscher Brutvogelarten. Atlas of German breeding birds. Stiftung Vogelmonitoring Deutschland, Dachverband Deutscher Avifaunisten, Münster
Green RE (1999) Applications of large-scale studies of demographic rates to bird conservation. Bird Study 46:279–288. https://doi.org/10.1080/00063659909477255
Grueber CE, Nakagawa S, Laws RJ, Jamieson IG (2011) Multimodel inference in ecology and evolution: challenges and solutions. J Evolution Biol 24:699–711. https://doi.org/10.1111/j.1420-9101.2010.02210.x
Gurnell J, Flowerdew JR (2006) Live trapping small mammals: a practical guide. Mammal Society, London
Hardey J, Crick H, Wernham C, Riley H, Etheridge B, Thompson D (2013) Raptors: a field guide for surveys and monitoring. Third edition, TSO
Harrell E, Lee KL, Mark DB (1996) Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Statist Med 15:361–387
Holt DW (1992) Notes on Short-eared Owl, Asio flammeus, nest sites, reproduction, and territory sizes in coastal massachusetts. Ca Field-Nat 106:352–356
Huang K, Gauthier P, Karpik J (2010) Short-eared owl (Asio flammeus) and Townsend's vole (Microtus Townsendii) dynamics in grassland Set-asides. Fish, Wildlife & Recreation; British Columbia Institute of Technology
IPCC (2021) Summary for policymakers. In: Climate change 2021: the physical science basis
Jareño D, Viñuela J, Luque-Larena JJ, Arroyo L, Arroyo B, Mougeot F (2014) A comparison of methods for estimating common vole (Microtus arvalis) abundance in agricultural habitats. Ecol Indic 36:111–119. https://doi.org/10.1016/j.ecolind.2013.07.019
Johnson DH, Swengel SR, Swengel AB (2013) Short-eared Owl (Asio flammeus) occurrence at Buena Vista Grassland, Wisconsin, during 1955–2011. J Raptor Res 47:271–281. https://doi.org/10.3356/JRR-12-00006.1
Kamp J, Frank C, Trautmann S, Busch M, Dröschmeister R, Flade M, Gerlach B, Karthäuser J, Kunz F, Mitschke A, Schwarz J, Sudfeldt C (2021) Population trends of common breeding birds in Germany 1990–2018. J Ornithol 162:1–15. https://doi.org/10.1007/s10336-020-01830-4
Kämpfer S, Oberdiek N, Dierschke J (2013) Nistplatzwahl von Sumpfohreulen Asio flammeus auf Spiekeroog im Nationalpark Niedersächsisches Wattenmeer. Vogelkundl Ber Niedersachs 43:241–250
Kämpfer, S, Fartmann, T (2020) Status and trend of the short-eared owl in Germany. In: Bos J, Schaub T, Klaassen R, Kuiper M, (eds.) Book of abstracts. International Hen Harrier and Short-eared Owl meeting 2019, Groningen
Kämpfer S, Engel E, Hirschberg M. Klock M, Fartmann T (2020) Breeding and feeding ecology of the short-eared owl on the East Frisian Islands (NW Germany). In: Bos J, Schaub T, Klaassen R, Kuiper M (eds) Book of abstracts. International Hen Harrier and Short-eared Owl meeting (2019) Groningen
Keller V, Herrando S, Voříšek P, Franch M, Kipson M, Milanesi P, Martí D, Anton M, Klvañová A, Kalyakin MV, Bauer H-G, Foppen RPB (2020) European breeding bird atlas 2: distribution. Abundance and Change. European Bird Census Council & Lynx Edicions, Barcelona
Kenward RE (2001) A manual for wildlife radio tagging. Academic, London
Kleefstra R, Barkema L, Venema DJ, Spijkstra-Scholten W (2015) Een explosie van Veldmuizen, een invasie van broedende Velduilen in Friesland in 2014. Limosa 88:74–82
Klock M (2018) Aktivitätsmuster, Fütterungsfrequenz und Beutespektrum der Sumpfohreule (Asio flammeus) auf den Ostfriesischen Inseln. Bachelor-Thesis, Georg-August-Universität Göttingen, Analyse von Nestkameradaten
Knipping N, Hallmann C, Reichert G, Südbeck P, Stahl J (2020) Breeding Hen Harriers in the German Wadden Sea - long-term research documents steep decline. In: Bos J, Schaub T, Klaassen R, Kuiper M (Eds) Book of abstracts. International Hen Harrier and Short-eared Owl meeting 2019. Groningen
Korpimäki E, Norrdahl K (1991) Numerical and functional responses of kestrels, short-eared owls, and long-eared owls to vole densities. Ecology 72:814–826. https://doi.org/10.2307/1940584
Kouba M, Bartoš L, Tomášek V, Popelková A, Šťastný K, Zárybnická M (2017) Home range size of Tengmalm’s owl during breeding in Central Europe is determined by prey abundance. PLoS ONE 12:e0177314. https://doi.org/10.1371/journal.pone.0177314
Krüger T (2019) Sumpfohreulen Asio flammeus als Brutvögel in Mähwiesen: Gefährdung und Schutz. Vogelwelt 139:183–201
Laake J (2020) Package ‘RMark’. R package version 2.2.7. https://cran.r-project.org/web/packages/RMark/index.html
Ludwig SC, Aebischer NJ, Bubb D, Roos S, Baines D (2018) Survival of chicks and adults explains variation in population growth in a recovering red grouse Lagopus lagopus scotica population. Wildlife Biol. https://doi.org/10.2981/wlb.00430
Martínez DR, Figueroa RA, Ocampo CL, Jaksic FM (1998) Food habitats and hunting ranges of short-eared Owls (Asio flammeus) in agricultural landscapes of Southern Chile. J Raptor Res 32(2):111–115
Masson-Delmotte V, Zhai P, Pirani A, Connors SL, Péan C, Berger S, Caud N, Chen Y, Goldfarb L, Gomis MI, Huang M, Leitzell K, Lonnoy E, Matthews JBR, Maycock TK, Waterfield T, Yelekçi O, Yu R, Zhou B (eds) (2022) Contribution of working group I to the sixth assessment report of the intergovernmental panelon climate change. Cambridge University Press, Cambridge
Miller RA, Battistone C, Hayes H, Courtney JC, Meyer A, Tisdale C, Larson MD, Barnes JG, Armstrong W, Alexander JD, Paprocki N, Hansen A, Pope TL, Norvell R, Buchanan JB, Lee M, Carlisle JD, Moulton CE, Booms TL (2020) Short-eared owl population site, distribution, habitat use, and moddeled response to changing climate: 2020 annual and comprehensive report. Western Asio Flammeus Landscape Study (WafLS)
Millon A, Petty SJ, Little B, Gimenez O, Cornulier T, Lambin X (2014) Dampening prey cycle overrides the impact of climate change on predator population dynamics: a long-term demographic study on tawny owls. Glob Chang Biol 20:1770–1781. https://doi.org/10.1111/gcb.12546
Nakagawa S, Johnson PCD, Schielzeth H (2017) The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J R Soc Interface 14:1–11. https://doi.org/10.1098/rsif.2017.0213
Newton I (2017) Farming and Birds. William Collins, London
Nuijten RJM, Vriend SJG, Wood KA, Haitjema T, Rees EC, Jongejans E, Nolet BA (2020) Apparent breeding success drives long-term population dynamics of a migratory swan. J Avian Biol. https://doi.org/10.1111/jav.02574
PECBMS (2020) Common farmland bird indicator, PanEuropean common bird monitoring scheme. https://pecbms.info/trends-and-indicators/indicators/indicators/E_C_Fa. Accessed 25 January 2021
Petersen J, Kers B, Stock M (2014) TMAP-typology of coastal vegetation in the Wadden Sea Area. Common Wadden Sea Secretariat (CWSS), Wilhelmshaven
Petersen J, Pott R (2005) Ostfriesische Inseln. Landschaft und vegetation im Wandel. Schrift Heimatpfl Niedersächs Heimatb 15:1–160
Pitelka FA, Tomich PQ, Treichel GW (1955) Ecological relations of jaegers and owls as lemming predators near barrow, alaska. Ecol Monogr 25:85–117
R Development Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reif J, Hanzelka J (2020) Continent-wide gradients in open-habitat insectivorous bird declines track spatial patterns in agricultural intensity across Europe. Glob Ecol Biogeogr 29:1988–2013. https://doi.org/10.1111/geb.13170
Reynolds P, Gorman ML (1999) The timing of hunting in short-eared owls (Asio flammeus) in relation to activity patterns of Orkney voles (Mictotus arvalis orcadensis). Zool (london). https://doi.org/10.1111/j.1469-7998.1999.tb01000.x
Roos S, Smart J, Gibbons DW, Wilson JD (2018) A review of predation as a limiting factor for bird populations in mesopredator-rich landscapes: a case study of the UK. Biol Rev 93:1915–1937. https://doi.org/10.1111/brv.12426
Ryslavy T, Bauer H-G, Gerlach B, Hüppop O, Stahmer J, Südbeck S, Sudfeldt C (2021) Rote Liste der Brutvögel Deutschlands—6. Fassung, 30. September 2020. Ber Zum Vogelschutz 57:13–112
Schaub T, Klaassen R (2020) Movements and diet of short-eared owls in the Netherlands. In: Bos J, Schaub T, Klaassen R, Kuiper M (eds) Book of abstracts. International Hen Harrier and short-eared owl meeting 2019, Groningen
Schöll EM, Hille SM (2020) Heavy and persistent rainfall leads to brood reduction and nest failure in a passerine bird. J Avian Biol 51:e02418. https://doi.org/10.1111/jav.02418
Škorpíková V, Horal D, Štěpánek P, Berka P (2020) A breeding invasion of the short-eared owl (Asio flammeus) into South and Central Moravia in 2019. Crex 38:24–43 ((In Czech with English abstract))
Smith AC, Hudson MAR, Aponte V, Francis CM (2019) North American breeding bird survey - Canadian trends website, data-version 2017. Environment and Climate Change Canada, Gatineau
Steen DA, Gibbs JP (2004) Effects of roads on the structure of freshwater turtle populations. Conserv Biol 18:1143–1148. https://doi.org/10.1111/j.1523-1739.2004.00240.x
Stokes MK, Slade NA, Blair SM (2001) Influences of weather and moonlight on activity patterns of small mammals: a biogeographical perspective. Can J Zool 79:966–972. https://doi.org/10.1139/z01-059
Swengel SR, Swengel AB (2014) Short-eared owl abundance and conservation recommendations in relation to site and vegetative characteristics, with notes on Northern Harriers. Passeng Pigeon 76:51–68
Tatner P (1989) Energetic demands during brood rearing in the wheatear Oenanthe oenanthe. Ibis 132:423–435. https://doi.org/10.1111/j.1474-919X.1990.tb01060.x
Van Manen W (2001) Influence of weather circumstances on behaviour and hunting success of wintering long-eared owls Asio otus. Limosa 74:81–86
Van Turnhout CAM, Foppen RPB, Leuven RSEW, van Strien A, Siepel H (2010) Life-history and ecological correlates of population change in Dutch breeding birds. Biol Conserv 143:173–181. https://doi.org/10.1016/j.biocon.2009.09.023
Vickery WL, Bider JR (1981) The influence of weather on rodent activity. J Mammal 62:140–145. https://doi.org/10.2307/1380484
Walter G, Kleinekuhle J (2008) Die Landsäuger der Ostfriesischen Inseln. In: Niedringhaus R, Haeseler V, Janisch P (eds) Die Flora und Fauna der Ostfriesischen Inseln: Artenverzeichnisse und Auswertungen zur Biodiversität. Schriftenr Nationalp Niedersächs, Wattenm, pp 441–449
White GS, Garrot AG (1990) Analysis of wildlife radio-tacking data. Academic Press
Wiggins D (2004) Short-eared Owl (Asio flammeus): a technical conservation assessment. USDA Forest Service, Rocky Mountain Region. https://www.fs.usda.gov/Internet/FSE_DOCUMENTS/stelprdb5182042.pdf Assessed 01 Feb 2021
Wróbel A, Bogdziewicz M (2015) It is raining mice and voles: which weather conditions influence the activity of Apodemus flavicollis and Myodes glareolus? Eur J Wildl Res 61:475–478. https://doi.org/10.1007/s10344-014-0892-2
Acknowledgements
We are grateful to Johanna Falk, Edgar Schonart, Melinda Hirschberg and Melanie Klock for support during field work. Additionally, we would like to thank the NLWKN in Norden, especially Martin Schulze-Dieckhoff, Josephin Erber and Joachim Ihnken for the provision of data and general support and all volunteers for assistance during the search for nests. Moreover, we are grateful to Nadine Knipping for logistic and organisational assistance and the Wadden Sea National Park Administration of Lower Saxony, particularly Peter Südbeck, Gundolf Reichert and Bernd Oltmanns for their support. We are grateful to two anonymous reviewers for valuable comments on an earlier version of the manuscript.
Funding
The study was funded by a Ph.D. scholarship from the German Federal Environmental Foundation (DBU) and research funding by the German Ornithologists’ Society (DO-G). Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Ethical approval
The National Park was accessed under licence of the National Park Administration of the Wadden Sea National Park of Lower Saxony [01.1-22242/23-1.0(6-14)]. Permits for catching, ringing and radio tagging were granted by the Lower Saxony Water Management, Coastal Defence and Nature Conservation Agency (D7.2220/VB_2018) and the Lower Saxony State Office for Consumer Protection and Food Safety (33.19-42502-04-18/2786).
Additional information
Communicated by O. Krüger.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kämpfer, S., Engel, E. & Fartmann, T. Weather conditions determine reproductive success of a ground-nesting bird of prey in natural dune grasslands. J Ornithol 163, 855–865 (2022). https://doi.org/10.1007/s10336-022-01999-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-022-01999-w