Abstract
Microgeographic variation in bird song has been studied in many species. However, it remains to be more fully described in birds that do not learn song and that thus have largely innate vocalisations. In this study, we focused on microgeographic variation in the territorial calls of one such non-learning species, the Corncrake (Crex crex). We examined the temporal and spectral characteristics of male Corncrake calls recorded in five separate populations in Poland. We found significant but weak relationships between some of the call characteristics and between-caller distances in four populations. The patterns observed were inconsistent. For some call characteristics, similarity decreased as distance between callers increased, while the opposite was true for other characteristics. Moreover, the same call characteristics showed opposite patterns in different populations or even within the same population but in different years. These findings suggest that the specific spatial distribution of the different quality habitats in which populations are located may have a crucial influence on microgeographic variation in calls. Alternatively, in non-learning species, various levels of microgeographic variation in calls might arise independently as a consequence of (1) social interactions among males, during which birds modify some parameters of their inherited call; or (2) territory occupancy patterns that minimize or maximize call similarity among neighbours.
Zusammenfassung
Mikrogeographische Unterschiede in den Rufen einer nicht-lernenden Art, dem Wachtelkönig
Mikrogeographische Unterschiede im Gesang von Vögeln wurden in vielen Arten untersucht. Sie müssen jedoch eingehender beschrieben werden für Arten, die ihren Gesang nicht lernen und daher größtenteils angeborene Lautäußerungen haben. In dieser Untersuchung konzentrierten wir uns auf mikrogeographische Unterschiede in den Territorialrufen einer solchen Art, dem Wachtelkönig (Crex crex). Wir untersuchten die zeitlichen und spektralen Eigenschaften von Rufen männlicher Wachtelkönige, aufgenommen in fünf verschiedenen Populationen in Polen. Wir fanden signifikante aber schwache Beziehungen zwischen einigen der Eigenschaften der Rufe und dem Abstand zwischen den Rufern in vier Populationen. Die beobachteten Muster waren inkonsistent. Für einige der Rufeigenschaften nahm die Ähnlichkeit mit steigendem Abstand zwischen den Rufern ab, während für andere das Gegenteil der Fall war. Darüber hinaus zeigten dieselben Rufeigenschaften gegensätzliche Muster in unterschiedlichen Populationen oder sogar innerhalb derselben Population in unterschiedlichen Jahren. Diese Ergebnisse legen nahe, dass die spezifische räumliche Verteilung der Habitate von unterschiedlicher Qualität, in der sich die verschiedenen Populationen befinden, einen entscheidenden Einfluss haben könnte auf mikrogeographische Unterschiede in den Rufen. Alternativ könnten verschiedene Ausprägungen von mikrogeographischen Unterschieden zwischen den Rufen nicht-lernender Arten unabhängig zutage treten als Folge von (1) sozialen Interaktionen zwischen Männchen, in denen die Vögel einige Parameter ihrer angeborenen Rufe anpassen, oder (2) von Mustern der Besetzung von Territorien, welche die Ähnlichkeit von Rufen zwischen Nachbarn minimieren oder maximieren.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microgeographic variation in bird song describes the differences in the vocalisations produced by individuals that are separated by varying degrees of distance within the dispersal range of a given species (i.e. individuals that could potentially interact or interbreed with each other) (Mundinger 1982). This kind of variation has been studied in many species, and various spatial patterns have been observed (for a review, see Catchpole and Slater 2008; Podos and Warren 2007). For example, in the Cinnamon-breasted Bunting (Emberiza tahapisi) individuals share more song types with their neighbours than with their non-neighbours (Osiejuk 2011). The opposite pattern has been observed in the Common Chaffinch (Fringilla coelebs), where individuals share fewer song types with neighbours than with non-neighbours (Lachlan and Slater 2003). In other species, such as the Tree Pipit (Anthus trivialis), song similarity among neighbours is equivalent to that observed among individuals located a few territories apart (Petrusková et al. 2010). In Dupont’s Lark (Chersophilus duponti), a large degree of local variation in song has been observed in some populations, while other populations display more vocal homogeneity (Laiolo and Tella 2005). In the White-crowned Sparrow (Zonotrichia leucophrys), local variation in song is consistent across time (Harbison et al. 1999), whereas in the Wedge-tailed Sabrewing (Campylopterus curvipennis), there is temporal variability, with patterns that appear and disappear over short time periods (Gonzalez and Ornelas 2005).
This huge variability of microgeographic song variation in time and space can be explained by two interacting factors: a song learning and a dispersion pattern of a given species. Song learning in birds has been observed only in songbirds (Oscines), Parrots (Psittaciformes), and Hummingbirds (Trochillidae) (reviewed in Catchpole and Slater 2008). In this process, an individual listens and copies particular songs or phrases which are sung by conspecifics. The timing of song acquisition is species specific, and in most birds called closed-ended learners, is short, and usually occurs only in the first year of their life (Beecher 1996). However, some species, the open-ended learners, are able to modify their repertoire and acquire new songs or phrases also in adulthood (Todt and Geberzahn 2003; Araya-Salas and Wright 2013). Therefore, the main influence on local song variation is likely the dispersion pattern and the site fidelity of a particular species or population, from which, or where, offspring (or in some species, also adult individuals) learn their songs, and how individuals disperse in the first and further years of their life.
Non-learning species may also exhibit microgeographic call variation, which can be associated with call characteristics that are constrained by genetics and morphology (i.e. the anatomy of the sound-producing apparatus). Thus, microgeographic variation in non-learners may be shaped by (1) the spatial distribution of differently calling individuals, or by (2) social interactions among conspecifics, during which birds modify some parameters of their inherited call [e.g. rhythm, song rate, intensity or start of singing (Ręk 2013; Snijders et al. 2015)]. In the first case, significant differences in call characteristics between neighbours and distant birds would suggest that males occupy territories in such a way as to minimize or maximize call similarity among neighbours. The most interesting question is whether such local variation in non-learners has any biological function (males intentionally establish territory near to similarly/differently singing conspecific) or is it just a side effect of habitat structure (males take into account different factors than call similarity to potential neighbour when they establish a territory). In the second case, local song variation would be based on social interactions. Even simple and inherited signals of non-learning birds could be broadcasted at varying intensity, rhythm or syntax (Ręk and Osiejuk 2010, 2013). Therefore, individuals may modify their inherited acoustic signal to be more or less similar to surrounding males influenced by interactions with conspecifics (Ręk 2013; Snijders et al. 2015).
Unfortunately, in non-learning species, geographic patterns of song variation have been rarely studied and, when they have been, mainly at a macrogeographic scale (e.g. Budka et al. 2014; Ippi et al. 2011; Isler et al. 2005; Lovell and Lein 2013; Odom and Mennill 2012; Peake and McGregor 1999; Walcott et al. 2006). Probably, such differences in studies on learners and non-learners are due to an assumption that species which do not show vocal learning do not show microgeographic variation, i.e. varying types of songs are haphazardly distributed in space. Consequently, we do not know much about microgeographic variation in the calls of non-learners; in particular, it is unclear which spatial pattern is the most common and which factors influence and maintain microgeographic variation in this group of birds (Catchpole and Slater 2008).
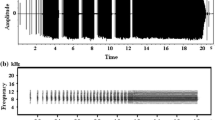
In this study, we focused on microgeographic variation in the calls of a non-learning species, the Corncrake (Crex crex) (Brenowitz 1991). Corncrakes inhabit meadows and grasslands in Eurasia (Berg and Gustafson 2007; Budka and Osiejuk 2013a; Cramp and Simmons 1980) and winter in southern Africa (Walther et al. 2013). During the breeding season, males are vocally active at night, when they produce their characteristic, monotonous call (Green et al. 1997). The cracking call of the Corncrake is the functional equivalent of a songbird’s song because it is used to attract mates and deter rivals (Cramp and Simmons 1980; Schäffer 1995). It has a very simple structure and consists of two syllables separated by an interval (Fig. 1). Birds can change the duration of the syllables (S1 and S2) and the within- (I1) and between-call intervals (I2) over short time periods; however, any changes made to S1, S2, and I1 are rather minor (Budka and Osiejuk 2013b; Osiejuk et al. 2004; Ręk and Osiejuk 2010, 2013). The durations of the intervals and the proportion of the different syllables uttered define rhythm [RTM = I2/(S1 + I1 + S2) (Osiejuk et al. 2004)]. Call rhythm is a standard signal that contains information about male aggressiveness and quality, and it may change over the course of social interactions (Ręk and Osiejuk 2010, 2013). Male Corncrakes are able to interpret a signal’s temporal pattern and, following aggressive interactions with other males, acquire new signalling strategies that they then use to convey their aggressiveness (Ręk 2013). Consequently, social interactions in the Corncrake, and likely in other non-learning species, may be responsible for local variation in non-innate calls, just as social interactions shape song rate and start time of singing in songbirds (Snijders et al. 2015). In contrast, the intervals between successive maximum-amplitude peaks within each syllable, called pulse-to-pulse durations (PPDs) (for further PPDs; see Fig. 1), are static: they are stable over a bird’s life and appear to be highly individual (Peake et al. 1998; Budka et al. 2015). This call characteristic is likely heritable and is strongly constrained by the anatomy of the sound-production apparatus. Therefore, local variation in PPD is predicted to be shaped by bird distribution patterns and environmental factors that influence bird development. Like PPD, the energy distribution across the signal’s frequency range should serve as an honest signal. In animals, the size of the vocal tract determines the lower frequencies of the signal (Bradbury and Vehrencamp 2011), and vocal tract length is positively correlated with body size (Fitch 2000). Therefore, larger individuals should produce lower frequency signals than smaller individuals (Bradbury and Vehrencamp 2011). For this reason, variation in the energy distribution across the frequency range is predicted to reflect the male size distribution.
Corncrake call parameters measured: duration of the first syllable (S1), duration of the second syllable (S2), duration of the within-call interval (I1), duration of the between-call interval (l2), frequency below which 25% of the total signal energy is distributed (L25). Each syllable usually consists of 15–22 repeated maximal amplitude peaks. Number of pulses within the first syllable fewer than within the second syllable. Rhythm of calling is defined as I2/(S1 + I1 + S2). PPD Pulse-to-pulse duration
In this study, we focused on microgeographic variation in the temporal and spectral characteristics of the calls produced by male Corncrakes in different populations. We split analysed call characteristic into two groups: relatively individually constant [S1, S2, I1, PPD, frequency below which 25% acoustic energy of the signal is distributed (L25)], and functionally variable (I2, RTM). Our null hypothesis was that call characteristics would demonstrate no microgeographic patterns, and as a consequence, neighbours and distant birds would not differ in their calls. Alternatively, we expected that habitat structure (homogeneity or heterogeneity) within a population, dominant hierarchy of calling males, or intentional territory occupancy based on call similarity to that of a potential neighbour could cause one of the following spatial patterns of call similarity: (1) a positive spatial pattern, where similarity is greater among neighbouring than among distant individuals; or (2) a negative spatial pattern, where similarity is greater among distant than among neighbouring males.
Materials and methods
Study populations and call-recording methodology
Calling male Corncrakes (n = 233) belonging to five populations in Poland were recorded during the 2010–2012 breeding seasons (Table 1). These populations were located in important bird areas of international importance. In Poland, Corncrakes inhabit meadows, grasslands, abandoned areas, and pastures varying in agricultural intensity (Wilk et al. 2010). The distance between populations ranged from 187 to 571 km. Males were non-uniformly distributed, and the average distance to the nearest neighbour within populations ranged from 204 to 464 m. The population in Kampinoski National Park was sampled twice, once in 2010 and once in 2011. All call recordings were made at night (2200–0400 hours, local time) from 24 May to 30 June. In each population, we conducted 6–15 successive nights of recording. The distance between the microphone and the bird varied from a few metres to approximately 15 m. Recordings were made using a Marantz PMD 620 recorder and a Sennheiser ME67 directional microphone with a K6 powering module. All recordings were of the same quality (44.1 kHz/16 bit). Representative sequences of all recordings are deposited in the Animal Sound Archive at the Museum für Naturkunde, Berlin (www.animalsoundarchive.org). The recordings are freely accessible as files Crex_crex_89354 until Crex_crex_89586. We did not individually mark the birds we recorded. Thus, to avoid recording the same male twice, we attempted to record all males present within each suitable habitat patch over the course of a single night. The exact location of each calling male was determined using a global positioning system (GPS) receiver. All the GPS data were transferred from the receiver to a desktop personal computer and were saved using the WGS 84 coordinate system. The distances between individuals and populations were calculated using ArcGis version 9.3 software with Hawth’s analysis tools for ArcGis (Beyer 2004).
Call analysis and data selection
For our analyses, we chose a continuous series of 20 calls without any significant background noise to represent each male. The temporal and spectral characteristics of the calls were quantified using Avisoft SASLab Pro version 5.2.04 software (Specht 2014). All the data are illustrated in Fig. 1.
To quantify the temporal characteristics (S1, S2, I1, and I2), we used the following set of parameter values: fast Fourier transform length = 512, frame = 25%, window = Hamming, and temporal overlap = 87.50%. These settings resulted in a 448-Hz bandwidth with a frequency of 86 Hz and a time resolution of 1.45 ms. We also calculated call rhythm [RTM = I2/(S1 + I1 + S2)].
L25 was measured using a one-dimensional function called amplitude spectrum (linear) and employing a Hamming evaluation window. The spectral characteristics option ‘total’ was activated. To remove background noise, the minimum frequency was limited to 0.5 kHz (Osiejuk and Olech 2004).
PPD structure and the number of pulses within the first (NPS1) and second (NPS2) syllables were quantified using the pulse train analysis function. Before PPD was measured, all sounds were high-pass filtered with a FIR time-domain filter (0.5 kHz) to remove low-frequency noises. We used the rectification + exponential decay method to measure PPD. For the pulse train analysis, we initially used the following parameter values: time constant = 1 ms, threshold = 0.10 V, hysteresis = 10 dB, and start end threshold = −8 dB. However, for a few recordings, we had to decrease the threshold and vary hysteresis between 9 and 12 dB to detect all of the pulses. The pulse distributions were visually examined to verify that all the pulses had been detected. Additional technical details and an explanation of the Avisoft SASLab Pro settings used in this study can be found in Specht (2014).
Statistical analyses
First, based on the 20 calls measured for each individual, we calculated the average values of all the call characteristics for each male, which were then used in further analyses. L25 values were highly and significantly correlated between the first and second syllables (Pearson’s correlation, r = 0.996, p < 0.01). Therefore, we only retained the characteristics of the first syllable in our analyses. In this study, the smallest number of pulses for both the first and second syllables was 15. However, the first 14 PPDs were highly correlated between the first and second syllables (Pearson’s correlation, average r = 0.981). Therefore, we only used the first 14 PPDs of the first syllable in our analyses. This approach makes sense because the last PPDs within the syllable are randomly distributed and do not demonstrate a clear individual pattern (Peake et al. 1998).
In our analyses of microgeographic call variation, we determined whether differences in call characteristics were distance dependent. Each population was examined separately, and recordings made in different years in Kampinoski National Park were treated separately as well. We performed a series of simple Mantel tests (20,000 randomisations) to determine whether, within populations, call similarity decreased as the distance between callers increased. To this end, we compared the matrix of the between-individual distances (in metres) with the matrix of dissimilarity (Euclidean distances) for each call characteristic. Statistical analyses were performed using Rundom Pro version 3.14 (Jadwiszczak 2009). We analysed each call characteristic separately because principal component analysis does not allow for stepwise variable selection (Kaiser–Meyer–Olkin measure of sampling adequacy was 0.396). All p-values reported are two tailed.
Results
We analysed calls from 233 male Corncrakes from five populations in Poland. Each analysed male had at least one neighbour within hearing range (<1000 m). Descriptive statistics for each call characteristic for each population are provided in Table 2. The Mantel tests revealed significant but weak relationships between some of the call characteristics and between-caller distances in four populations (Table 3). The patterns observed were inconsistent. Dissimilarity in S1, S2, NPS1, NPS2 significantly decreased as the distance between callers increased only in one population. This result means that these call characteristics were more similar among neighbouring individuals than among distant birds. For I1, RTM and L25 the pattern was population dependent: dissimilarity both increased and decreased as the distance between callers increased, meaning that, in one population, neighbours were more similar in I1, RTM and L25 while, in another population, more distant birds were more similar in these call characteristics. The completely random pattern across all studied population was observed in two call characteristics: I2 and 14PPD (Table 3).
Discussion
Our study reports that non-learning bird species may also show various patterns of microgeographic variation in some call characteristics. In this group of birds microgeographic variation in their calls has been poorly studied. Trainer and Parsons (2001) examined microgeographic variation in vocalisations in a suboscine species, the Long-tailed Manakin (Chiroxiphia linearis), but did not observe any spatial pattern. Galeotti et al. (1996) found that the Tawny Owl (Strix aluco) exhibited microgeographic variation in its hoots and suggested that habitat structure was the explanatory factor for this. Peake and McGregor (1999) found a specific pattern of microgeographic variation in Corncrake calls. They analysed PPD variation at a microgeographic scale in one Irish (43 males) and two Scottish (14 and 36 males) subpopulations. They showed consistent patterns of microgeographic variation in all studied subpopulations–neighbouring individuals shared significantly more similar call structures than more distant birds. In our study, we discovered the following:
-
1.
Calls can be more similar among neighbouring than among distant males.
-
2.
Calls can be more similar among distant than among neighbouring males.
-
3.
Both neighbouring and distant males may not display any differences in call structure.
-
4.
The same call characteristic can show opposite spatial patterns in different populations, or even within the same population but in different years.
In non-learning species, microgeographic variation in calls can be shaped by two factors: social interactions among males, and the spatial distribution of differently calling males. Corncrakes, like other non-learning species, cannot learn new calls. They can, however, modify some call characteristics, like call rate or intensity. The rhythm of Corncrake calling is a characteristic which could be shaped by social interactions. RTM is strongly seasonally and daily changeable; however, differences between nights are significantly larger than within a single night (Osiejuk et al. 2004). Male Corncrakes can change RTM over the course of aggressive interactions (Budka and Osiejuk 2013b; Ręk and Osiejuk 2010). After an aggressive interaction with another male, male Corncrakes can signal their aggressiveness using the new rhythm pattern (Ręk 2013). Therefore, neighbouring males should establish a spatial pattern of rhythm that reflects their actual aggressive motivation within a local group. We found that, in two populations, dissimilarity in RTM decreased with increasing distance between individuals, while in a third we observed the opposite pattern. Moreover, the opposite pattern was observed within the same population (Kampinoski National Park) in different years (Table 3). This suggests that males interact with each other and that the microgeographic variation in RTM is a result of their social interactions. The lack of a consistent pattern across all populations and between population in different years may be caused by different habitat structure, agricultural usage, distance to nearest neighbours or population density in a particular year. Such differences in spatial variability of males and habitat structure may cause changes in call-distance dependency of a linear vs. a non-linear pattern. We assumed that dependence of call characteristics on distance among birds was approximately linear. This assumption may reduce the effectiveness of the Mantel test when the spatial pattern is more complex (Gloslee and Urban 2007). Thus, our approach to the analysis of microgeographic variation in the call of the Corncrake is conservative. The other Corncrake call characteristics examined here, such as S1, S2, I1, 14 PPDs, NPS1, NPS2 and L25, are rather static, and males can modify them only slightly (Osiejuk and Olech 2004; Osiejuk et al. 2004). Therefore, microgeographic variation in these call characteristics should be shaped solely by the spatial distribution of differently calling males. Males could occupy territories completely randomly or in such a way as to minimize or maximize call similarity among neighbours. For instance, males could occupy territories next to other males with different calls, so as to highlight their differences; this strategy is employed by some learning species (Wilson and Vehrencamp 2001). The opposite pattern also occurs, i.e. some birds appear to prefer a high degree of call similarity within local groups as it enables neighbour–stranger discrimination (Briefer et al. 2008; Radford 2005). Thus, individuals singing similar songs are identified as belonging to the local group. In our study, the individually consistent Corncrake call characteristics—S1, S2, NPS1, NPS2—were significantly more similar among neighbouring than among distant males only within one population. However, we found that similarity in I1 increased or decreased with increasing distance between individuals, dependent on population. This finding suggests that a group signature based on S1, S2, NPS1, NPS2 and I1 is not an important part of neighbour–stranger discrimination in this species, and that the birds did not take into consideration similarity of their calls to those of potential neighbours when settling territories.
Microgeographic variation in calls in non-learners could be also explained by the spatial distribution of different-quality habitats and, as a consequence, the distribution of different-quality males. Animals commonly use calls as reliable signals of sender body size (Bradbury and Vehrencamp 2011). In birds, the call characteristics that serve this purpose are still a point of debate (see for example Fitch 1999; Hall et al. 2013; Martin et al. 2011). However, it remains likely that, within species, larger individuals produce lower-frequency calls than smaller individuals (Bradbury and Vehrencamp 2011). A weak negative correlation between body size and L25 (r = −0.34, p = 0.017) was also found in the Corncrake (Osiejuk and Olech 2004). In Corncrake, larger individuals simultaneously occupy higher quality patches of habitat (Keiss et al. 2004). Therefore, mosaic patterns of habitat structure within population ranges could be crucial in shaping local variation in calls, particularly in spectral characteristics. Our results confirm this hypothesis, since we found that L25 was more similar among neighbouring than among distant individuals in one population, while in two others we found the opposite pattern. A habitat-related mechanism could also shape microgeographic call variation in RTM. Then, optimal patches (i.e. with tall and dense vegetation, where females are numerous) could be occupied by aggressive, rhythmically calling males, while suboptimal patches could be occupied by non-aggressive, monotonously calling individuals. In both cases, similarity in RTM and L25 would depend on the spatial distribution of different-quality habitat patches. Thus, opposite patterns could be expected in different populations or even in different years within the same population.
Inconsistent patterns of microgeographic variation found in both temporal and spectral Corncrake call characteristics suggest that local environmental factors, for example the spatial distribution of different-quality habitats, could strongly influence spatial patterns of call characteristics. Thus, microgeographic variation in Corncrake calls seems to be a side effect of the spatial distribution of various quality habitats. Alternatively, in non-learning species, various levels of microgeographic variation in calls might arise independently as a consequence of social interactions among males or territory occupancy patterns that minimize or maximize call similarity among neighbours.
References
Araya-Salas M, Wright T (2013) Open-ended song learning in a Hummingbird. Biol Lett 9:20130625
Beecher MD (1996) Birdsong learning in the laboratory and field. In: Kroodsma DE, Miller EH (ed) Ecology and evolution of acoustic communication in birds. Cornle University Press
Berg Å, Gustafson T (2007) Meadow management and occurrence of Corncrake Crex crex. Agric Ecosyst Environ 120:139–144
Beyer HL (2004) Hawth’s analysis tools for ArcGIS. Available at web site: http://www.spatialecology.com/htools
Bradbury JW, Vehrencamp SL (2011) Principles of animal communication, 2nd edn. Sinauer Associates, Sunderland
Brenowitz EA (1991) Evolution of the vocal control system in the avian brain. Sem Neurosci 3:339–407
Briefer E, Aubin T, Lehongre K, Rybak F (2008) How to identify dear enemies: the group signature in the complex song of the Skylark Alauda arvensis. J Exp Biol 211:317–326
Budka M, Osiejuk TS (2013a) Habitat preferences of Corncrake (Crex crex) males in agricultural meadows. Agric Ecosyst Environ 171:33–38
Budka M, Osiejuk TS (2013b) Neighbour–stranger call discrimination in a nocturnal rail species, the Corncrake Crex crex. J Ornithol 154:685–694
Budka M, Mikkelsen G, Turčoková L, Fourcade Y, Dale S, Osiejuk TS (2014) Macrogeographic variation in the call of the Corncrake Crex crex. J Avian Biol 45:65–74
Budka M, Wojas L, Osiejuk TS (2015) Is it possible to acoustically identify individuals within a population? J Ornithol 156:481–488
Catchpole CK, Slater PJB (2008) Bird song: biological themes and variations, 2nd edn. University Press, Cambridge
Cramp S, Simmons KEL (1980) The birds of the Western Palearctic, vol 2. University Press, Oxford
Fitch WT (1999) Acoustic exaggeration of size in birds via tracheal elongation: comparative and theoretical analyses. J Zool 248:31–48
Fitch WT (2000) Skull dimensions in relation to body size in nonhuman primates: the causal bases for acoustic allometry. Zoology 103:40–58
Galeotti PR, Appleby BM, Redpath SM (1996) Macro and microgeographical variation in the ‘hoot’ of Italian and English Tawny Owls (Strix aluco). Ital J Zool 63:57–64
Gloslee SC, Urban DL (2007) The ecodist package for dissimilarity-based analysis of ecological data. J Stat Softw 22:1–19
Gonzalez C, Ornelas JF (2005) Song structure and microgeographic song variation in Wedle-tailed Sabrewings (Campylopterus curvipennis) in Veracruz, Mexico. Auk 122:593–607
Green ER, Rocamora G, Schäffer N (1997) Populations, ecology and threats to the Corncrake Crex crex in Europe. Vogelwelt 118:117–134
Hall ML, Kingma SA, Peters A (2013) Male songbird indicates body size with low-pitched advertising songs. PLoS One 8:e56717
Harbison H, Nelson DA, Hahn TP (1999) Long-term persistence of song dialects in the mountain White-crowned Sparrow. Condor 101:133–148
Ippi S, Vásquez RA, van Dongen WFD, Lazzoni I (2011) Geographical variation in the vocalizations of the suboscine Thorn-tailed Rayadito Aphrastura spinicauda. Ibis 153:789–805
Isler ML, Isler PR, Brumfield RT (2005) Clinal variation in vocalizations of an Antbird (Thamnophilidae) and implications for defining species limits. Auk 122:433–444
Jadwiszczak P (2009) Rundom pro 3.14. Software for classical and computer-intensive statistics available free from the new rundom site (http://pjadw.tripod.com)
Keiss O, Granats J, Mednis A (2004) Use of biometrical data to study Corncrake Crex crex population in Latvia. Acta Univ Latv B 676:119–126
Lachlan RF, Slater PJB (2003) Song learning by chaffinches: how accurate, and from where? Anim Behav 65:957–969
Laiolo P, Tella JL (2005) Habitat fragmentation affects cultural transmission: patterns of song matching in Dupont’s Lark. J Appl Ecol 42:1183–1193
Lovell SF, Lein MR (2013) Geographical variation in songs of a suboscine passerine, the Alder Flycatcher (Empidonax alnorum). Wils J Ornithol 125:15–23
Martin JP, Doucet SM, Knox RC, Mennill DJ (2011) Body size correlates negatively with the frequency of distress calls and songs of Neotropical birds. J Field Ornithol 82:259–268
Mundinger PC (1982) Microgeographic and macrogeographic variation in the acquired vocalizations of birds. In: Kroodsma DE, Miller EH (eds) Ecology and evolution of acoustic communication in birds. Cornell University Press, Ithaca, pp 147–208
Odom KJ, Mennill DJ (2012) Inconsistent geographic variation in the calls and duets of Barred Owls (Strix varia) across an area of genetic introgression. Auk 129:298–387
Osiejuk TS (2011) The song of the Cinnamon-breasted Bunting, Emberiza tahapisi, in the Bamenda highlands (NW Cameroon). J Ornithol 152:651–659
Osiejuk TS, Olech B (2004) Amplitude spectra of Corncrake calls: what do they signalise? Anim Biol 54:207–220
Osiejuk TS, Olech B, Ratyńska K, Owsiński A, Gromadzka-Ostrowska J (2004) Effects of season, plasma testosterone and body size on Corncrake (Crex crex) call rhythm. Ann Zool Fenn 41:647–659
Peake TM, McGregor PK (1999) Geographical variation in the vocalization of the Corncrake Crex crex. Ecol Evol Ethol 11:123–137
Peake TM, McGregor PK, Smith KW, Tyler G, Gilbert G, Green RE (1998) Individuality in Corncrake Crex crex vocalization. Ibis 140:120–127
Petrusková T, Osiejuk TS, Petrusek A (2010) Geographic variation in songs of the Tree Pipit (Anthus trivialis) at two spatial scales. Auk 127:274–282
Podos J, Warren PS (2007) The evolution of geographic variation in birdsong. Adv Stud Behav 37:403–458
Radford AN (2005) Group-specific vocal signatures and neighbour–stranger discrimination in the cooperatively breeding Green Woodhoopoe. Anim Behav 70:1227–1234
Ręk P (2013) Concrake males acquire new signal meanings during aggressive interactions. Anim Behav 86:451–457
Ręk P, Osiejuk TS (2010) Sophistication and simplicity: conventional communication in a rudimentary system. Behav Ecol 21:1203–1210
Ręk P, Osiejuk TS (2013) Temporal patterns of broadcast calls in the Corncrake encode information arbitrarily. Behav Ecol 24:547–552
Schäffer N (1995) Calling behaviour and functions of calls in the Corncrake Crex crex. Vogelwelt 116:141–151
Snijders L, van der Eijk J, van Rooij EP, de Goede P, van Oers K, Naguib M (2015) Song trait similarity in Great Tits varies with social structure. PLoS One 10:e0116881
Specht R (2014) Avisoft-SASLab pro version 5.2, sound analysis and synthesis laboratory. Avisoft Bioacustics, Berlin
Todt D, Geberzahn N (2003) Age-dependent effects of song exposure: song crystallization set a boundary between fast and delayed vocal imitation. Anim Behav 65:971–979
Trainer JM, Parsons RJ (2001) Uniformity of long-tailed Manakin songs from three localities in Costa Rica. Wils Bull 113:431–434
Walcott C, Mager JN, Pipper W (2006) Changing territories, changing tunes: males Loons, Gavia immer change their vocalisatios when they change territories. Anim Behav 71:673–683
Walther BA, Taylor PB, Schaffer N, Robinson S, Jiguet F (2013) The African wintering distribution and ecology of the Corncrake Crex crex. Bird Conserv Int 23:309–322
Wilk T, Jujka M, Krogulec J, Chylarecki P (2010) Important bird areas of international importance in Poland. OTOP, Marki
Wilson PL, Vehrencamp SL (2001) A test of the deceptive mimicry hypothesis in song-sharing Song Sparrows. Anim Behav 62:1197–1205
Acknowledgements
We thank Paweł Ręk, Sylwester Solaniuk, and Krzysztof Jurczak for their help in the field, and Amie Wheeldon and an anonymous reviewer for their helpful comments on an earlier version of this manuscript. This study was partially funded by a grant from the dean of the Biology Department at Adam Mickiewicz University (GDWB-02/2011).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Naguib.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Budka, M., Osiejuk, T.S. Microgeographic call variation in a non-learning species, the Corncrake (Crex crex). J Ornithol 158, 651–658 (2017). https://doi.org/10.1007/s10336-017-1438-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-017-1438-7