Abstract
Recent studies have documented a mismatch between the phenology of leaf production, prey availability and the nestling food requirements of north temperate songbirds, attributed to climate change effects. Although tropical forest species have often been regarded as relatively aseasonal breeders, similar disruptive effects can be expected at equatorial latitudes, where comparatively little is known of the links between weather, leafing phenology, food availability and bird breeding activity, particularly in complex rain forest habitats. During a 19-year study at 1°S in Bwindi Impenetrable Forest, Uganda, Stripe-breasted Tits Parus fasciiventer showed a strongly bimodal laying pattern, breeding mainly in the two dry seasons, with 50 % of breeding activity occurring in January–February and 19 % in June–July. Individual females bred in both dry seasons, laying their first and last clutches up to 28 weeks apart. Breeding activity was linked to leaf production, which peaked mainly in November–December, following the September–November wet season. Increased leaf production is likely to have stimulated a rise in caterpillar numbers during December–February, coinciding with peak food demands by nestling tits. Laying was thus positively correlated with increased leaf production in the preceding calendar month, but was also linked to day length and a change in sunset time. To investigate possible links between egg laying and photic cues I compared the median date of first clutches laid by marked females in each half of the breeding year (October–March and April–September), with annual changes in photoperiod (varying by 7 min p.a.) and sunrise time (varying bimodally, by 31 min p.a.). The two median laying dates fell 138–139 days after the last date on which sunrise had occurred at 07:05 in August and January, suggesting the potential for sunrise time to act as a cue, or Zeitgeber, for breeding in tropical birds. Further work is required to establish whether the relationship is causative or coincidental.
Zusammenfassung
Niederschlagsmenge, Belaubungsphänologie und die Sonnenaufgangszeit als potenzieller Zeitgeber für das zweigipflige Eiablagemuster zur Trockenzeit bei einer Meisenart ( Parus fasciiventer ) afrikanischer Regenwälder Neuere Untersuchungen belegen ein Missverhältnis zwischen Phänologie der Laubproduktion, Beuteverfügbarkeit und Nahrungsbedarf der Nestlinge bei Singvögeln in nördlichen gemäßigten Breiten, was den Auswirkungen des Klimawandels zugeschrieben wird. Obgleich Vogelarten tropischer Wälder häufig als relativ nichtsaisonale Brutvögel betrachtet werden, sind ähnliche Störwirkungen in Äquatornähe zu erwarten, von wo vergleichsweise wenig über das Zusammenspiel von Wetter, Belaubungsphänologie, Nahrungsverfügbarkeit und der Brutaktivität von Vögeln bekannt ist, speziell in komplexen Regenwaldhabitaten. Während einer 19-jährigen Studie bei 1° südlicher Breite im Bwindi Impenetrable Forest, Uganda, zeigten Schwarzbrustmeisen Parus fasciiventer ein deutlich zweigipfliges Legemuster und brüteten hauptsächlich zu den beiden Trockenzeiten, wobei 50 % der Brutaktivitäten im Januar-Februar und 19 % im Juni-Juli stattfanden. Einzelne Weibchen brüteten in beiden Trockenperioden und produzierten ihre ersten und letzten Gelege im zeitlichen Abstand von bis zu 28 Wochen. Die Brutaktivität stand mit der Laubproduktion im Zusammenhang, welche ihren Höhepunkt hauptsächlich im November-Dezember erreichte, im Anschluss an die Regenzeit von September–November. Es ist wahrscheinlich, dass die erhöhte Laubproduktion einen Anstieg der Raupenzahlen von Dezember-Februar auslöste, was mit dem Spitzennahrungsbedarf der Meisennestlinge zusammenfiel. Somit korrelierte die Eiablage positiv mit der gesteigerten Laubproduktion des vorhergehenden Kalendermonats, stand aber ebenso mit der Tageslänge und einer Änderung der Sonnenuntergangszeit im Zusammenhang. Um möglichen Zusammenhängen zwischen der Eiablage und Helligkeitssignalen nachzugehen, wurden die mittleren Erstlegedaten markierter Weibchen aus beiden Hälften des Brutjahres (Oktober-März und April-September) mit jährlichen Änderungen der Photoperiode (sieben Minuten Abweichung pro Jahr) und der Sonnenaufgangszeit (zweigipflige Abweichung um 31 Minuten pro Jahr) verglichen. Die beiden mittleren Legedaten fielen 138–139 Tage nach dem letzten Datum, zu dem der Sonnenaufgang im August bzw. Januar um 07:05 Uhr stattfand, was die Sonnenaufgangszeit zu einem potenziellen Signal oder Zeitgeber für die Brut tropischer Vögel macht. Weitere Untersuchungen sind nötig um zu klären, ob diese Beziehung ursächlich ist oder auf Zufall beruht.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mechanism by which insectivorous songbirds time their breeding activity to coincide with peaks in prey availability has received considerable attention in Europe and North America, much of it focused on cavity nesters, particularly the tits (Paridae) and Ficedula flycatchers (e.g., Lack 1966; van Noordwijk et al. 1995; Both et al. 2004; Ramsay and Otter 2007). In north temperate deciduous woodlands the timing of leaf production (bud burst) is advanced by warm temperatures, as are the hatching dates and growth rates of leaf-eating caterpillars (Perrins 1979), which exploit the availability of tender, relatively tannin-free young leaves (Feeny 1970). Temperature is also used as a cue by north temperate tit species, whose breeding activity is broadly stimulated by increasing day length, and fine-tuned by food supply (Nilsson 1994) and ambient temperature (Nager and van Noordwijk 1995; Cresswell and McCleery 2003; Schaper et al. 2012). In some years at least, these adjustments ensure that the maximum food demands of most broods coincide with a peak in local prey availability.
This general pattern has been studied in considerable detail (e.g., Lack 1966; Perrins 1979; Blondel et al. 1990, 2006; Nager and van Noordwijk 1995; Ramsay and Otter 2007; Lehmann et al. 2012), enabling researchers to examine the effects of climate change on breeding seasonality and population dynamics at north temperate latitudes (Visser et al. 1998, 2003; Sæther et al. 2003; Both et al. 2004; Nussey et al. 2005; Visser et al. 2006; Charmantier et al. 2008; Visser et al. 2010; Reed et al. 2013; Gienapp et al. 2014), where the two main proximate cues for egg laying—day length and temperature—vary markedly throughout the year. Most passerines live at tropical or sub-tropical latitudes, however, where seasonal variation in these cues is much less pronounced and breeding activity is often considered to be relatively aseasonal, particularly in rain forest habitats. They include most members of the genus Parus, 65 % of which are endemic to sub-Saharan Africa (Gosler and Clement 2007).
Throughout Africa, Parus species occupy a broad range of woodland types, in which the timing of leaf and insect production is often positively related to rainfall (Moreau 1950; Sinclair 1978; Brown and Britton 1980). Consequently, most African Parids and other insectivores breed during the annual or biannual wet seasons (Moreau 1950; Brown and Britton 1980; Tarboton 1981; Fry et al. 2000; Wiggins 2001) or, in two cases, before the wet season begins (Brown and Britton 1980), when a sharp rise in ambient temperatures triggers bud burst in the Brachystegia-Julbernardia (miombo) woodland they occupy (Moreau 1950). A third general pattern is evident in Afromontane regions, where forest passerines may show a reversal of the “normal” response to rainfall, breeding instead during relatively dry months, perhaps to avoid the lower temperatures associated with high rainfall (Serle 1981; Tye 1992; Fotso 1996).
At equatorial latitudes the timing and volume of rainfall would appear to be the main factor influencing the timing of breeding in songbirds (Moreau 1950; Brown and Britton 1980; Radford and Du Plessis 2003; Styrsky and Brawn 2011; Oppel et al. 2013). While climate change is likely to have a disruptive effect on rainfall patterns in parts of tropical Africa, attempts to model climate change impacts on tropical birds have been hampered by a lack of long-term empirical data, and of information on species interactions (Harris et al. 2011; Şekercioğlu et al. 2012). These deficits are especially acute in rain forest habitats, where tree species diversity is high, the majority of species are evergreen, and the phenology of leaf and fruit production may be particularly complex.
As a breeding stimulus for tropical birds, photoperiod has received less attention than rainfall pattern, mainly for two reasons. First, it has been assumed that species close to the equator (e.g., at ±5° latitude) are incapable of detecting day length differences of just a few minutes over the course of the year (Voous 1950; Miller 1959). Second, while circannual variation in day length is unimodal, some tropical bird species show distinctly bimodal laying patterns (e.g., Brown and Britton 1980). Despite these observations, there is evidence that changes in photoperiod could influence breeding activity even at equatorial latitudes. Hau et al. (1998) have shown that Spotted Antbirds Hylophylax naevioides, at 9°N in Panama, are capable of detecting day length variation of as little as 17 min. And, importantly, Goymann et al. (2012) have demonstrated that moult patterns in captive African Stonechats Saxicola torquatus axillaris are linked to seasonal changes in the timing of sunrise (hereafter referred to as solar time), rather than variation in day length. The significance of this finding is twofold. First, the timing of sunrise and sunset at low latitudes varies annually with greater amplitude than day length change, providing a more easily detectable cue for equatorial species. Second, at low latitudes, sunrise and sunset times follow a bimodal pattern, with a periodicity of 6 months; roughly congruent with that of many bimodal breeders in equatorial Africa. Furthermore, a similar periodicity has been described in the flowering patterns of equatorial rain forest trees, broadly coinciding with peaks in the rate of change in sunrise and sunset times (Borchert et al. 2005). Hence, there is strong evidence that equatorial rain forest trees, and at least one bird species, are capable of responding to seasonal changes in solar time, rather than day length.
To identify the climatic and biotic factors associated with breeding activity in an equatorial passerine I monitored the timing of laying in the Stripe-breasted Tit P. fasciiventer, a species endemic to montane rain forests of the Albertine Rift in central Africa. Over a 19-year period, the tit’s breeding activity was recorded in Bwindi Impenetrable Forest, SW Uganda, where, at 1°S, seasonal variation in day length and temperature is much less pronounced than at north temperate latitudes. Moreover, since most of Bwindi’s tree and shrub species are evergreen, leaf replacement does not vary seasonally with the same amplitude or synchrony as in temperate deciduous woodlands. Consequently, seasonal peaks in the availability of caterpillars, accounting for 72 % of items provisioned to Stripe-breasted Tit broods (Shaw et al. 2015), are also likely to be less pronounced than at temperate latitudes, perhaps explaining the tit’s smaller brood sizes and protracted breeding pattern. At Bwindi, Stripe-breasted Tits have been recorded laying in 11 calendar months, the majority of broods being raised during the two annual dry seasons (Shaw et al. 2015).
Here, I examine the relationship between breeding activity by Stripe-breasted Tits and seasonal variation in weather, photic cues and leafing phenology. I determined whether marked individuals bred in both dry seasons, and whether those laying early were more likely to produce a second clutch during the same season. I predicted that the species’ bimodal, dry season breeding pattern would be correlated with tree leafing patterns during the preceding months, and that unusually high rainfall during the wet season would stimulate increased breeding activity during the following dry season. Finally, I consider whether seasonal variation in solar time has the potential to act as a synchronizing cue (or Zeitgeber) for egg laying in this species.
Methods
Study area
This study was conducted at the Institute of Tropical Forest Conservation (ITFC) field station at Ruhija, Bwindi Impenetrable Forest, SW Uganda (29°46′E, 1°02′S; c. 2330 m a.s.l.). The forest covers c. 331 km2 and comprises a mosaic of closed canopy areas with open, disturbed patches, the latter mainly on steep ridges and hills. A total of 324 tree and shrub species have been recorded (Davenport et al. 1996). Rainfall at Ruhija averaged 1374 mm p.a. during 1987–2012 (ITFC, unpublished) and is strongly bimodal, peaking in September–November and March–May, with dry periods in January–February and June–July, the latter being more pronounced (Fig. 1a). There is little seasonal variation in temperature, with mean monthly maxima of 18.2–19.8 °C and minima of 13.4–14.5 °C (Fig. 1b). While day length varies by 7 min. p.a. (Fig. 1c), the timing of sunrise and sunset varies by 31 min. p.a., and follows a distinctly bimodal pattern (Fig. 1d).
Seasonal variation in rainfall, temperature, day length, sunrise and sunset times at Ruhija, Bwindi Impenetrable Forest, 1995–2014. a Mean rainfall per day. b Mean maximum temperature (solid line) and mean minimum temperature (dotted line). c Day length (in min.). d The timing of sunrise (solid line) and sunset (dashed line) expressed as minutes after midnight (from USNO 2010)
Data collection
During 1996–2014 laying dates were recorded or estimated for 96 Stripe-breasted Tit clutches, all but three of which were laid in nestboxes (initially 25 boxes, rising to 80 by 2008). Boxes were inspected in most, but not all months during 1995–2000 and at least once in every month during 2001–2014. Active nests were checked or watched at 2–3 day intervals and daily at around the anticipated dates of laying, hatching and fledging. In most cases these events were recorded accurately to the day. Where laying dates were missed they were estimated by back-tracking from the hatching or fledging date, based on mean incubation and nestling periods (15.1 and 23.5 days; Shaw et al. 2015). A high proportion of clutches were laid in December–January, with smaller numbers in October–November, preceded by a period of courtship, site selection and nest building. Hence, 1 October was taken as the start of the breeding year (Shaw et al. 2015).
Daily rainfall and minimum and maximum temperatures were recorded at Ruhija manually (1987–2012) and by an automatic weather station located c. 3 km from the study site (2011–2014). Since readings were sometimes missed, the total rainfall for a given month was estimated by multiplying the mean daily rainfall by the number of days in the month. This assumes that rainfall itself did not influence the likelihood of a day being missed. Months in which weather data were collected on fewer than 80 % of days were excluded from the analyses. Day lengths and the timing of sunrise and sunset (to the nearest minute) were downloaded from USNO (2010).
It was not possible to monitor seasonal variation in insect abundance, due to financial constraints. Tree and shrub leafing phenology data were made available from two studies, however. The Gorilla Food Plant Study (GFPS: 2004–2013) monitored 319 individuals of 32 tree and shrub taxa known to feature in the diet of a Mountain Gorilla Gorilla beringei graueri population at Bwindi (M. Robbins pers. comm. 2014). The Extended Phenology Study (EPS: 2011–2013) monitored 529 individuals of 52 taxa (R. Barigyira pers. comm. 2014). Individual plants were assessed monthly on the following scale. 0: absence of new leaves; 1: 1–10 new leaves; 2: 10–100; 3: 100–1000; 4: >1000. In combination, the two studies made 43,582 assessments of 60 tree and shrub taxa. Of these, 18 were excluded from the analysis because they could not be identified to species level, or because sample sizes were considered too small (<10 observations per calendar month) (ESM Table 1).
Data analysis
I compared Stripe-breasted Tit breeding activity in each month with measures of rainfall, temperature, day length, sunrise and sunset times, and leaf production. Two measures of breeding activity were examined: the number of clutches initiated each month, as a precise measure of the onset of breeding; and the number of brood-days recorded, i.e. the number of days on which broods were in the nest in each month, summed for all broods. Thus, if two broods were in the nest in a given month, each for 20 days, a score of 40 was recorded. This provided an indication of the timing of peak food requirements in the study population. These comparisons were made on two levels: in relation to calendar months (data pooled by calendar month, across all years) and year-months (data analysed in relation to specific months and years).
Calendar month analysis
I calculated the mean rainfall per day and mean maximum and minimum temperatures for each calendar month, from all months in which these parameters were recorded on at least 80 % of days, during 1995–2014 (Fig. 1a, b). I calculated the mean leafing score for each tree or shrub species in each calendar month during 2004–2013, and identified the three highest-scoring calendar months for each species. Leaf production was defined as “high” for the species in question during these three calendar months. I then determined the number of species for which leaf production was high in each calendar month. I used the lm command in R (3.0.1; R Development Core Team 2009) to examine the relationship between the mean number of clutches initiated per day in each calendar month, the number of species showing high leafing scores, and the mean rainfall, temperature, day length, sunrise time and sunset time recorded. Each explanatory variable was expressed as a proportion of the highest value in any calendar month. Since the conditions that stimulate breeding are likely to precede egg-laying by several weeks, I also compared the number of clutches initiated per day with leafing, weather and photic values from the previous calendar month (i-1) and from two months previously (i-2). To minimise the number of terms used in each model, I initially compared the dependent variable with one potential explanatory variable in three forms, e.g. with rainfall in month i, month i-1 and month i-2. From each group I selected the version showing the strongest correlation with the number of clutches initiated per day, and ran a full model in which the following variables were included: number of species for which leaf production was high; maximum and minimum temperatures; rainfall; day length; sunrise and sunset times. I used the R step command to sequentially eliminate non-significant terms whose removal from the model reduced the Akaike Information Criterion (AIC) value by <2, leaving a final, minimal model. The R plot, qqnorm and hist functions were used to determine whether final models reasonably met with model assumptions (Crawley 2013).
Year-month analysis
I used the glmer function in the lme4 package in R (3.0.1; R Development Core Team 2009) to fit generalised linear mixed models (GLMMs) to investigate the relationship between each breeding parameter and potential explanatory variables, in each year-month. Since a small number of breeding attempts may have been missed in some year-months prior to 2003–04, the analyses were restricted to October 2003 to December 2013.
To determine whether the level of breeding activity in each month was correlated with leafing phenology I first used Principal Component Analysis (PCA) to identify groups of plant species showing similar seasonal patterns of leaf production, using the prcomp function in R. I compared the results from PC analyses made using data from the two phenology schemes combined and from the GFPS on its own. I used GLMMs to investigate the relationship between measures of breeding activity in each month and scores for the first three principal components from each dataset. Both datasets spanned 10 years, in which the same months were represented. Since AIC values from models incorporating data from the GFPS only were, in every case, lower than those incorporating data from both schemes, only the GFPS data series was used subsequently when comparing breeding activity with leafing phenology. Models incorporating leafing phenology data were thus further restricted, to September 2004–September 2013.
Because no breeding attempts were made in a high proportion of year-months, the distribution of each response variable (clutches initiated or brood-days recorded) was highly skewed. I therefore examined the relationship between breeding activity and potential explanatory variables using two model structures. First, I identified explanatory variables associated with the presence/absence of breeding attempts in each year-month, specifying a binomial error distribution. In the second model I restricted the dataset to year-months in which at least one breeding attempt had occurred (i.e. a clutch was initiated or brood-days recorded, as appropriate) and specified a Poisson error distribution. In each model type “study year” and “calendar month” were entered as random variables. Fixed variables were selected using the same approach as in the calendar month analysis; each dependent variable was compared with versions of a given explanatory variable, e.g. rainfall in month i, month i-1 and month i-2, and the version showing the strongest correlation with the dependent variable was selected. The full models thus included one version of each potential explanatory variable: mean rainfall, temperature, day length, sunrise time, sunset time and leaf production score. Minimal models were derived through stepwise elimination of the least significant fixed variables. Final models were those with the lowest AIC value.
The potential influence on breeding activity of abiotic and biotic variables was examined initially in separate models, since the number of values missing from each dataset, combined with the short time span for which biotic data were available, would have severely restricted the sample of cases that could be included in each model. I then examined the effects on breeding activity of biotic and abiotic variables in combination, using only those variables whose effects had been significant in either of the two previous series of models.
Laying patterns of individuals
I examined the laying dates and breeding success of individual females known to have survived from October to August in a given breeding year (n = 13 females; 31 female-years), to determine whether the timing and frequency of laying during the first dry season influenced breeding performance during the remainder of the year. Specifically, I recorded whether individuals bred in both dry seasons, whether early-laying females produced more clutches during the year, and whether the number, timing or success of breeding attempts made in October–February influenced the number of clutches laid in March–September. I used GLMMs to investigate the latter, specifying a binomial or Poisson error distribution to examine the occurrence and number of breeding attempts made, respectively. Since the dataset included repeated measures from the same individuals and study years, “female identity” and “study year” were entered as random factors. Laying dates were expressed relative to the start date of the season.
To determine whether day length or the pattern of change in solar time (after Goymann et al. 2012) could act as a cue for egg laying, I calculated the median dates on which clutches were initiated by individually marked females in each half of the breeding year (1 October–31 March, 1 April–30 September; n = 17 females; 46 clutches) and compared these with seasonal changes in day length and solar time. The analysis was restricted to first clutches in each half-year, since the timing of any subsequent clutches (in the same half-year) is likely to vary stochastically, depending on the duration and fate of the first attempt.
Whole-season analyses
To examine the relationship between weather variables and the level of breeding activity recorded in each season I compared the numbers of clutches laid, eggs laid and fledglings reared during each December–February dry season, with mean rainfall and temperatures recorded per day during the preceding September–November rains. The same comparison was made between each June–August dry season and the preceding the March–May rains, during 2004–2014.
All statistical tests were made using R (3.0.1; R Development Core Team 2009) or PASW® STATISTICS 19 software (SPSS Inc., Chicago, IL, USA). All probabilities are quoted as two-tailed.
Results
Breeding seasonality: calendar months
Stripe-breasted Tit clutches were initiated in 11 calendar months over the course of the study, but in just 2–7 months in any given year (median = 3 months; n = 11 years). Clutch initiations spanned a median of 26 weeks p.a. (range: 5–31 weeks; n = 11 years) and showed a strongly bimodal pattern, 51 % of clutch initiations (n = 96) and 69 % of brood-days (n = 1,514) occurring in the four driest calendar months (Fig. 2). Although breeding activity thus coincided with low rainfall, more brood-days were recorded during the January–February dry season (50 %) than in the (drier) June–July season (19 %).
Seasonal variation in Stripe-breasted Tit breeding activity, 1995–2014. a Clutches initiated per day in each half-month ( ), as a proportion of the maximum recorded in any half-month. The spread of clutches laid by two females (a and b), over the course of the same year, are shown as examples. b The number of brood-days recorded per day in each half-month (
), as a proportion of the maximum recorded in any half-month. The spread of clutches laid by two females (a and b), over the course of the same year, are shown as examples. b The number of brood-days recorded per day in each half-month ( ), as a proportion of the maximum brood-days recorded in any half-month. Monthly rainfall, as a proportion of the annual maximum, has been superimposed (grey line)
), as a proportion of the maximum brood-days recorded in any half-month. Monthly rainfall, as a proportion of the annual maximum, has been superimposed (grey line)
Individual females laid up to four clutches p.a., a given female initiating her first and last clutches up to 28 weeks apart. Individuals were thus capable of laying in both breeding seasons as well as during the intervening months (Fig. 2a). Those laying early in October–February were more likely to lay multiple clutches during this period than those laying later (multiple clutches = −0.142 (±0.069 SE) relative laying date +10.356 (±5.457 SE), z = −2.062, p = 0.039). However, neither the timing of laying during this period, the number of clutches laid, nor their outcome, influenced the number or timing of clutches laid in the following March–September.
Seasonal variation in leaf production varied markedly between tree species, both in terms of pattern and amplitude. In some species high leaf production coincided with, or closely followed, the September–November wet season; in others, it followed both wet seasons (ESM Fig. 1). Of 42 tree and shrub species monitored adequately, 35 (83 %) showed high leaf production during November–December, compared with only nine (21 %) in June–July (Fig. 3). For most species, new leaf production thus peaked during the two months preceding the January–February dry season, when brood-rearing also peaked (Fig. 2b).
The number of tree and shrub species showing high leafing scores in each month (mean scores in the upper quartile for that species), as a proportion of the highest species count in any calendar month. Leaf production was recorded during 2004–2013. Monthly rainfall, as a proportion of the annual maximum, has been superimposed (grey line)
A GLM comparing the number of clutches initiated per day in each calendar month with abiotic variables indicated that laying was positively related to day length two months previously and to changes in the timing of sunset since the previous month: clutches initiated per day = 17.407 (±5.211 SE) day length (i-2) + 20.053 (±4.084 SE) sunset change in past month −17.108, F 2,9 = 25.18, p < 0.0003; adjusted R 2 = 0.815. When leaf abundance (the number of species showing high leaf production in the previous month) was added to the model, all three variables were retained in a final, minimal model: clutches initiated per day = 0.013 (±0.004 SE) leaf abundance (i-1) + 13.274 (±4.105 SE) day length (i-2) + 9.896 (± 4.614 SE)sunset change in past month −13.098, F 2,8 = 33.55, p < 0.0001; adjusted R 2 = 0.899.
Breeding activity in specific months
The first three axes of a PCA of monthly leaf production scores explained 0.767, 0.061 and 0.031 of the variance, respectively. PC 1 represented the majority of species for which leaf production peaked mainly in November–December prior to the first dry season (ESM Fig. 2). Since subsequent axes individually explained only a very small proportion of the variance, only PC 1 was used in models combining abiotic and biotic factors. The presence/absence of clutch initiations in a given month was positively linked to leaf production (PC 1) scores 1 month earlier, and to an increase in the timing of sunrise over the previous month (Table 1). In contrast, the number of clutches initiated was positively linked to a rise in the mean minimum temperature over the previous two months (Table 1).
Sunrise and sunset peaks and troughs
Median dates on which first clutches were initiated in each half of the breeding year (1 October–31 March and 1 April–30 September) occurred in December and June, close to the longest and shortest days of the year. The median day length fell approximately midway between these laying dates, and thus preceded the median laying dates in December and June by a similar interval; by 80 and 81 days, respectively. The equinoxes, when the rate of change in day length peaks, also preceded the two median clutch initiation dates by broadly similar intervals: of 76 days (March equinox) and 91 days (September equinox). Accordingly, median day length, or a change in day length, could have the potential to act as a synchronizing cue for egg-laying.
To determine whether changes in the timing of sunrise or sunset might also have the potential to act as Zeitgeber I compared the median dates on which first clutches were initiated in each half-year with the timing of seasonal peaks in sunrise and sunset times. Lag-times between sunrise and sunset peaks and troughs, and subsequent median clutch initiation dates, all showed a marked disparity between the first and second half of the breeding year. A linear mixed-effects model, in which female identity and study year were entered as random variables, confirmed that lag times differed significantly with respect to half-year in all four cases (ESM Table 2), suggesting that seasonal peaks (or troughs) are unlikely to act as a cue for laying in both halves of the breeding year.
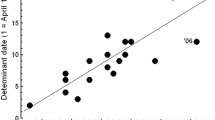
The disparity in lag-times may reflect the fact that sunrise (and sunset) peaks and troughs differ in magnitude. Thus, the peak value in October–March (431 min; 07:11) is never attained in April–September (maximum: 425 min; 07:05) (Fig. 4). The end of this second peak (i.e. the date after which sunrise time advanced by 1 min) preceded the next median laying date (on 23 December) by 138 days. During December–January sunrise time continued to increase, passing 425 min again 139 days before the next median laying date (on 4 June). Thus, the median laying dates on which first clutches were initiated in each half of the breeding year occurred 138–139 days after a point in the cycle at which sunrise last occurred at 07:05 in January and August (Fig. 4).
Median dates on which first clutches were initiated in October–March and April–September (filled squares), in relation to seasonal variation in the timing of sunrise (black line), expressed as minutes after midnight. In October–March the median laying date fell 138 days after the last day on which sunrise occurred at 425 min. Similarly, during April–September the median laying date fell 139 days after the last date on which the sunrise time had occurred at 425 min (grey line), and was increasing
Whole-season analyses
The numbers of eggs hatched and offspring fledged in each December–February dry season were positively correlated with rainfall level during the preceding September–November wet season. However, this relationship was significant only when a single outlier was excluded: December–February 2010–2011, during which breeding productivity was low, despite unusually heavy rainfall in the preceding wet season (Fig. 5). This may have been linked to an unusually productive season in June–August 2010, when the numbers of offspring hatched and fledged were, respectively, six and nine times the mean for that time of year. There was no relationship between temperature during September–November and breeding activity during December–February, nor between weather conditions during the March–May wet season and breeding activity in the June–August dry season.
Breeding productivity during each December–February dry season, in relation to mean daily rainfall during the preceding September–November wet season. Each point represents one study year. When one outlier (open symbol) was excluded, productivity increased significantly in relation to mean rainfall (mm per day) over the previous three months. a Eggs hatched = 5.642 ± 2.047 SE(Rainfall) − 5.008 ± 7.622 SE; adjusted R 2 = 0.49; F 1,6 = 7.594; p = 0.033. b Fledglings = 5.462 ± 1.741 SE(Rainfall) − 8.233 ± 6.481 SE; adjusted R 2 = 0.56; F 1,6 = 9.843; p = 0.020
Discussion
Stripe-breasted Tits laid up to four clutches each year, aggregated mainly into two seasons, such that most broods were in the nest during the driest months of the year: in January–February and June–July. The tit’s protracted, bimodal, dry-season breeding pattern thus contrasts markedly with the short, unimodal breeding season of its temperate congeners and with that of its African congeners, most of which breed in lowland woodland or savanna habitats, during the wettest months of the year (Brown and Britton 1980; Fry et al. 2000).
Dry season breeding
Tropical rain forest birds have often been regarded stereotypically as relatively aseasonal breeders, despite geographically widespread evidence of pronounced seasonality, typically coinciding with high rainfall (Fogden 1972; Brown and Britton 1980; Hau et al. 1998; Wikelski et al. 2000; Oppel et al. 2013, Goymann and Helm 2015). Where there is marked seasonal variation in rainfall, increased leaf production and insect abundance generally occurs during the wet season(s) rather than the dry season(s) (Fogden 1972; Sinclair 1978; Wolda 1978, 1988; Novotny and Basset 1998; Struhsaker 1998; da Silva et al. 2011, but see Reich et al. 2004; Grøtan et al. 2012). Rainfall stimulates evergreen trees and shrubs to increase the volume of young leaves produced, providing a window of opportunity for Lepidoptera species and their predators, typically spanning 1–2 months (Coley 1983; Basset 1991; Intachat et al. 2001; Hopkins and Memmott 2003).
Why, then, do some Afromontane insectivores rear their broods during the single or twice-yearly dry seasons? One explanation is that heavy rainfall and low temperatures reduce insect prey activity, and hence increase the level of parental effort required to provision the brood (Avery and Krebs 1984; Radford et al. 2001). Heavy rainfall can also increase the risk of nests being flooded out (Wesołowski et al. 2002; Radford and Du Plessis 2003) or of nestlings becoming chilled, and has been shown to reduce nest survival in a montane, sub-tropical population of Green-backed Tits P. monticolus (Shiao et al. 2015). Although this effect has been proposed as an explanation for dry-season breeding in Afromontane rain forests (Tye 1992; Fotso 1996), at Bwindi more broods were reared during the January–February dry season than the (drier) June–July season, suggesting that additional factors apply.
At Bwindi, high rainfall in September–November (Fig. 1a) preceded a marked increase in new leaf production by tree and shrub species in November–December (Fig. 3), which in turn is likely to have stimulated a rise in caterpillar abundance over the following months, coinciding with peak brood-rearing by Stripe-breasted Tits in January–February (Fig. 2b). Fewer tree species showed increased leaf production during or just prior to the tit’s second, smaller breeding peak in June–August (Fig. 3), although six of the nine species that did so were considered to be common or very common in the study area (R. Barigyira pers. comm. 2014), perhaps having a disproportionate influence on caterpillar abundance.
Evidence of the impact of increased rainfall and leaf production on butterfly abundance in western Uganda has been presented in a detailed study at Kibale Forest, a mid-altitude rain forest c. 180 km north of Bwindi. Over a 12-year period Valtonen et al. (2013) monitored 100+ butterfly species at monthly intervals. As at Bwindi, precipitation at Kibale was higher during the August–November wet season than in March–May. Vegetation “greenness” peaked approximately 33 days after seasonal peaks in precipitation and, importantly, adult butterfly abundance peaked c. 3 months after each peak in greenness; in February and August. Since larval and pupal stages of common butterfly species at Kibale have been shown to average 36 and 14 days, respectively (Molleman et al. 2016), large, mature caterpillars are likely to have been most abundant in January–February and July–August; peak brood-rearing months for Stripe-breasted Tits at Bwindi (Fig. 2b).
It is likely that dry season breeding by Stripe-breasted Tits, and perhaps by other Afromontane rain forest insectivores, is simply a consequence of the 2–3 month lag between high rainfall and a rise in caterpillar abundance. Bimodal rainfall patterns, which are widespread in equatorial Africa, produce short, alternating wet and dry seasons, each lasting c. 3–4 months. Consequently, if most evergreen rain forest trees and shrubs respond to a peak in rainfall midway through each wet season, as shown here, the resulting increase in caterpillar numbers will necessarily occur mainly in the following dry season, as will most breeding attempts by rain forest insectivores. In contrast, wet season breeding is much more common among insectivores in semi-arid habitats throughout much of East Africa (Brown and Britton 1980), despite these being subject to a similar, bimodal cycle of short wet- and dry seasons. This disparity could reflect a difference in the response shown by plants in rain forest and semi-arid habitats if the latter respond to the first heavy rains marking the beginning of the wet season, incidentally allowing sufficient time for insect larvae and insectivore nestlings to pupate and fledge during the same season. The timing of peak food availability in lowland savannas might be further advanced if insect development is more rapid there than in (cooler) montane forests (B. Helm pers. comm.), and if flying insects are more prone to move rapidly into savanna areas following recent, heavy rainfall (e.g. Sinclair 1978).
Day length and solar time as potential Zeitgeber
For Stripe-breasted Tits day length and sunrise time each have the potential to act as Zeitgeber for egg-laying. Of the two candidate systems, day length presents the simpler, more parsimonious scenario; median first-laying dates in December and June were each preceded by a day length of 727 min, 80 and 81 days beforehand, respectively. For these events to act as Zeitgeber, however, Stripe-breasted Tits would have to be physiologically capable of distinguishing this day length from one differing by ± 3–4 min, i.e. ranging between 724 and 731 min throughout the year. This would exceed the level of sensitivity reported for other bird species to date, notably the 17 min change in photoperiod to which captive Spotted Antbirds have been shown to respond (Hau et al. 1998). Moreover, while the latter were stimulated consistently using artificial light, Stripe-breasted Tits in this study were subject to the potentially confounding effects of cloud cover. Through its effects on light intensity cloud cover can in itself act as a synchronizing cue for circannual rhythms (Gwinner and Scheuerlein 1998), and may effectively mask the very small changes in day length occurring at low latitudes (Dittami and Gwinner 1985). Photoperiod would, therefore, appear unlikely to yield a detectable cue for Stripe-breasted Tits, demanding a much greater sensitivity to day length change than has been demonstrated previously.
In contrast, the annual change in solar time varies by 31 min at Bwindi, cycling through twin peaks and troughs, each at differing levels (Fig. 1d). Goymann et al. (2012) recorded annual variation in solar time of the same amplitude and frequency at Nakuru, Kenya, and have demonstrated that African Stonechats Saxicola torquatus axillaris captured at the site were capable of detecting this pattern of change. Specifically, captive birds exposed to a constant equatorial day length, but with a simulation of the annual periodic change in sunrise and sunset times, began their single, annual moult 141 days after the higher of the two annual peaks in the timing of sunrise, and did so with greater synchrony than individuals subject to constant solar time (Goymann et al. 2012).
These findings suggest that Stripe-breasted Tits could be physiologically capable of detecting the pattern and amplitude of change in solar time experienced at Bwindi, and of using features of this cycle as Zeitgeber for egg-laying. However, there was no evidence that both sunrise peaks (or troughs) were used as cues for egg laying, since the lag time between each of these events and the subsequent median laying date of first clutches differed significantly between each half of the breeding year. Instead, Stripe-breasted Tits showed a more consistent delay between the absolute timing of sunrise, at 425 min, and egg laying. That is, the median date of first clutches fell 138–139 days after the last date on which this value was attained in August and again in January (Fig. 4).
This interval is likely to provide adequate time for gonadal development in Stripe-breasted Tits, given that the time required for testes expansion in a congener (the Great Tit P. major) is just c. 42 days (Silverin et al. 1993). Note, however, that both of these potential Zeitgeber occurred during or just after the two main breeding periods, requiring that individuals are sensitive to photic cues whilst breeding. That songbirds can remain sensitive to photic cues during the breeding season has been demonstrated by Pohl (1999) who showed that high Arctic passerines respond to changes in the spectral composition of sunlight during the summer. Also, Helm and Gwinner (2005) have shown that photostimulation during the spring can act as a cue for initiating events several months later, in the autumn. However, since sunrise at Bwindi occurs at 425 min in March also (Fig. 4), the scenario proposed here is tenable only if the March event coincides with a photorefractory period, during which the species is insensitive to sunrise time. The existence of photorefractory periods has been demonstrated in a range of songbirds (Dawson et al. 2001; Dawson 2008), including captive Stonechats of temperate and equatorial subspecies (S. t. rubicola and S. t. axillaris; Gwinner and Scheuerlein 1999). When exposed to day-length variations with an amplitude of 7 h, both subspecies switched from a state of refractoriness to photosensitivity at around moult completion (the timing of which has not been established for Stripe-breasted Tits). Gwinner and Scheuerlein (1999) also showed that equatorial Stonechats appeared to initiate refractoriness under shorter photoperiods, with an amplitude of 1 h 10 min, but concluded that it was unclear whether the much smaller photoperiodic changes are sufficient to synchronize circannual rhythms of equatorial birds.
In conclusion, whether features of solar time could be used as a cue for breeding by equatorial birds remains to be tested experimentally, although evidence presented here indicates that such a system could be tenable in the case of Stripe-breasted Tits breeding at 1°S.
References
Avery MI, Krebs JR (1984) Temperature and foraging success of Great Tits Parus major hunting for spiders. Ibis 126:33–38
Basset Y (1991) The seasonality of arboreal arthropods foraging within an Australian rainforest tree. Ecol Entomol 16:265–278
Blondel J, Perret P, Maistre M (1990) On the genetical basis of the laying-date in an island population of blue tits. J Evol Biol 3:469–475
Blondel J, Thomas DW, Charmantier A, Perret P, Bourgault P, Lambrechts MM (2006) A 30-year study of phenotypic and genetic variation of blue tits in Mediterranean habitat mosaics. Bioscience 56(8):661–673
Borchert R, Renner SR, Calle Z, Navarrete D, Tye A, Gautier L, Spichiger R, von Hildebrand P (2005) Photoperiodic induction of synchronous flowering near the Equator. Nature 433:627–629
Both C, Artemyev AV, Blaauw B, Cowie RJ, Dekhuijzen AJ, Eeva T, Enemar A, Gustafssons L, Ivankina EV, Järvinen AJ, Metcalfe NB, Erik N, Nyholm I, Potti J, Ravussin P-A, Sanz JJ, Silverin B, Slater FM, Sokolov LV, Török J, Winkel W, Wright J, Zang H, Visser ME (2004) Large-scale geographical variation confirms that climate change causes birds to lay earlier. Proc R Soc Lond B 271:1657–1662
Brown LH, Britton PL (1980) The breeding seasons of East African birds. East African Natural History Society, Nairobi
Charmantier A, McCleery RH, Cole LR, Perrins C, Kruuk LEB, Sheldon BC (2008) Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320(5877):800–803
Coley PD (1983) Herbivory and defensive characteristics of tree species in a lowland tropical forest. Ecol Monogr 53:209–234
Crawley MJ (2013) Statistics: an introduction to using R. Wiley, Chichester
Cresswell W, McCleery R (2003) How great tits maintain synchronization of their hatch date with food supply in response to long-term variability in temperature. J Anim Ecol 72:356–366
da Silva NAP, Frizzas MA, de Oliveira CM (2011) Seasonality in insect abundance in the “Cerrado” of Goiás State, Brazil. Revista Brasileira de Entomologia 55(1):79–87
Davenport T, Howard P, Matthews R (eds) (1996) Bwindi impenetrable national park biodiversity report. Report to the Forest Department, Kampala
Dawson A (2008) Control of the annual cycle in birds: endocrine constraints and plasticity in response to ecological variability. Phil Trans R Soc B 363:1621–1633
Dawson A, King VM, Bentley GE, Ball GF (2001) Photoperiodic control of seasonality in birds. J Biol Rhythms 16:365–380
Dittami J, Gwinner E (1985) Annual cycles in the African Stonechat Saxicola torquata axillaris and their relationship to environmental factors. J Zool 207:357–370
Feeny P (1970) Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 51:565–581
Fogden MPL (1972) The seasonality and population dynamics of equatorial birds in Sarawak. Ibis 114:307–343
Fotso RC (1996) Seasonal breeding in birds and its implications for the conservation of biodiversity in the Oku region, Cameron. Bird Conserv Int 6:393–408
Fry CH, Keith S, Urban EK (eds) (2000) The birds of Africa, vol VI. Academic Press, London
Gienapp P, Reed T, Visser ME (2014) Why climate change will invariably alter selection pressures on phenology. Proc R Soc Lond B 281:20141611. doi:10.1098/rspb.2014.1611
Gosler AG, Clement P (2007) Family Paridae (Tits and Chickadees). In: del Hoyo J, Elliott A, Christie DA (eds) Handbook of the Birds of the World, vol 12., Picathartes to Tits and Chickadees. Lynx Edicions, Barcelona, pp 662–750
Goymann W, Helm B (2015) Seasonality of life-cycles in tropical birds: circannual rhythms and Zeitgeber. In: Numata H, Helm B (eds) Annual, lunar and tidal clocks: patterns and mechanisms of nature’s enigmatic rhythms. Springer, Tokyo. ISBN 9784431552604
Goymann W, Helm B, Jensen W, Schwabl I, Moore IT (2012) A tropical bird can use the equatorial change in sunrise and sunset times to synchronize its circannual clock. Proc R Soc B 279:3527–3534
Grøtan V, Lande R, Engen S, Sæther B-E, DeVries PJ (2012) Seasonal cycles of species diversity and similarity in a tropical butterfly community. J Anim Ecol 81:714–723
Gwinner E, Scheuerlein A (1998) Seasonal changes in day-light intensity as a potential zeitgeber of circannual rhythms in equatorial Stonechats. J Ornithol 139:407–412
Gwinner E, Scheuerlein A (1999) Photoperiodic responsiveness of equatorial and temperate-zone stonechats. Condor 101:347–359
Harris JBC, Şekercioğlu CH, Sodhi NS, Fordham DA, Paton DC, Brook BW (2011) The tropical frontier in avian climate impact research. Ibis 153:877–882
Hau M, Wikelski M, Wingfield JC (1998) A Neotropical forest bird can measure the slight changes in tropical photoperiod. Proc R Soc Lond B 265:89–95
Helm B, Gwinner B (2005) Carry-over effects of day length during spring migration. J Ornithol 146:348–354
Hopkins GW, Memmott J (2003) Seasonality of a tropical leaf-mining moth: leaf availability versus enemy-free space. Ecol Entomol 28:687–693
Intachat J, Holloway JD, Staines H (2001) Effects of weather and phenology on the abundance and diversity of geometroid moths in a natural Malaysian tropical rain forest. J Trop Ecol 17:411–429
Lack D (1966) Population studies of birds. Oxford University Press, Oxford
Lehmann M, Spoelstra K, Visser ME, Helm B (2012) Effects of temperature on circadian clock and chronotype: an experimental study in a passerine bird. Chronobiol Int 29(8):1062–1071
Miller AM (1959) Reproductive cycles in an equatorial sparrow. Condor 61:344–347
Molleman F, Remmel T, Sam K (2016) Phenology of predation on insects in a tropical forest: temporal variation in attack rate on dummy caterpillars. Biotropica 48:229–236
Moreau RE (1950) The breeding seasons of African birds—1. Land birds. Ibis 92:223–267
Nager RG, van Noordwijk AJ (1995) Proximate and ultimate aspects of phenotypic plasticity in timing of great tit breeding in a heterogeneous environment. Am Nat 146:454–474
Nilsson J-Ǻ (1994) Energetic bottle-necks during breeding and the reproductive cost of being too early. J Anim Ecol 63:200–208
Novotny V, Basset Y (1998) Seasonality of sap-sucking insects (Auchenorrhyncha, Hemiptera) feeding on Ficus (Moraceae) in a lowland rain forest in New Guinea. Oecologia 115:514–522
Nussey DH, Postma E, Gienapp P, Visser ME (2005) Selection on heritable phenotypic plasticity in a wild bird population. Science 310:304–306
Oppel S, Hilton GM, Allcorn R, Fenton C, Matthews AJ, Gibbons DW (2013) The effects of rainfall on different components of seasonal fecundity in a tropical forest passerine. Ibis 155:464–475
Perrins CM (1979) British Tits. Collins, London
Pohl H (1999) Spectral composition of light as a Zeitgeber for birds living in the high Arctic Summer. Physiol Behav 67(3):327–337
R Development Core Team (2009) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Radford AN, Du Plessis MA (2003) The importance of rainfall to a cavity-nesting species. Ibis 145:692–694
Radford AN, McCleery RH, Woodburn RJW, Morecroft MD (2001) Activity patterns of parent Great Tits Parus major feeding their young during rainfall. Bird Study 48:214–220
Ramsay SM, Otter KA (2007) Fine-scale variation in the timing of reproduction in titmice and chickadees. In: Otter KA (ed) The ecology and behavior of Chickadees and Titmice. Oxford University Press, Oxford, pp 55–66
Reed T, Jenouvrier S, Visser ME (2013) Phenological mismatch strongly affects individual fitness but not population demography in a woodland passerine. J Anim Ecol 82(1):131–144
Reich PB, Uhl C, Walters MB, Prugh L, Ellsworth DS (2004) Leaf demography and phenology in Amazonian rain forest: a census of 40 000 leaves of 23 tree species. Ecol Monogr 74(1):3–23
Sæther B-E, Engen S, Møller AP, Matthysen E, Adriaensen F, Fiedler W, Leivits A, Lambrechts MM, Visser ME, Anker-Nilssen T, Both C, Dhondt AA, McCleery RH, McMeeking J, Potti J, Røstad OW, Thomson D (2003) Climate variation and regional gradients in population dynamics of two hole-nesting passerines. Proc R Soc B 270:2397–2404
Schaper SV, Dawson A, Sharp P, Gienapp P, Caro SP, Visser ME (2012) Increasing temperature, not mean temperature, is a cue for avian timing of reproduction. American Naturalist 179(2):E55–E69
Şekercioğlu CH, Primack RB, Wormworth J (2012) The effects of climate change on tropical birds. Biol Cons 148:1–18
Serle W (1981) The breeding season of birds in lowland rainforest and in the montane forest of west Cameroon. Ibis 123:62–74
Shaw P, Owoyesigire N, Ngabirano S, Ebbutt D (2015) Life history traits associated with low annual fecundity in a central African Parid: the Stripe-breasted Tit Parus fasciiventer. J Ornithol 156:209–221
Shiao M-T, Chuang M-C, Yuan H-W, Wang Y (2015) Effects of weather variation on the timing and success of breeding in two cavity-nesting species in a subtropical montane forest in Taiwan. Auk 132:671–684
Silverin B, Massa R, Stokkan KA (1993) Photoperiodic adaptation to breeding at different latitudes in great tits. Gen Comp Endocrinol 90(1):14–22
Sinclair ARE (1978) Factors affecting the food supply and breeding of resident birds and movements of Palearctic migrants in a tropical African savannah. Ibis 120:480–497
Struhsaker TT (1998) Ecology of an African Rain Forest. Logging in Kibale and the Conflict between Conservation and Exploitation. University Press of Florida, Gainesville, p 434
Styrsky JN, Brawn JD (2011) Annual fecundity of a Neotropical bird during years of high and low rainfall. Condor 113:194–199
Tarboton WR (1981) Cooperative breeding and group territoriality in the Black Tit. Ostrich 52:216–225
Tye H (1992) Reversal of breeding season by lowland birds at higher altitudes in western Cameroon. Ibis 134:154–163
USNO (2010) US Navy Oceanography Portal. http://aa.usno.navy.mil/data/. Accessed 30 Apr 2010
Valtonen A, Molleman F, Chapman CA, Carey JR, Ayres MP, Roininen H (2013) Tropical phenology: bi-annual rhythms and interannual variation in an Afrotropical butterfly assemblage. Ecosphere 4(3):1–28
van Noordwijk AJ, McCleery RH, Perrins CM (1995) Selection for the timing of great tit breeding in relation to caterpillar growth and temperature. J Anim Ecol 64(4):451–458
Visser ME, van Noordwijk AJ, Tinbergen JM, Lessells CM (1998) Warmer springs lead to mistimed reproduction in great tits (Parus major). Proc R Soc B 265:1867–1870
Visser ME, Adriaensen F, van Balen JH, Blondel J, Dhondt AA, van Dongen S, du Feu C, Ivankina EV, Kerimov AB, de Laet J, Matthysen E, McCleery R, Orell M, Thomson DL (2003) Variable responses to large-scale climate change in European Parus populations. Proc R Soc B 270:367–372
Visser ME, Holleman LJM, Gienapp P (2006) Shifts in caterpillar biomass phenology due to climate change and its impact on the breeding biology of an insectivorous bird. Oecologia 147:164–172
Visser ME, Caro SP, van Oers K, Schaper SV, Helm B (2010) Phenology, seasonal timing and circannual rhythms: towards a unified framework. Phil Trans R Soc B 365:3113–3127
Voous KH (1950) The breeding seasons of birds in Indonesia. Ibis 92:279–287
Wesołowski T, Czeszczewik D, Rowiński P, Walankiewicz W (2002) Nest soaking in natural holes—a serious cause of breeding failure? Ornis Fennica 79:132–138
Wiggins DA (2001) Low reproductive rates in two Parus species in southern Africa. Ibis 143:677–680
Wikelski M, Hau M, Wingfield JC (2000) Seasonality of reproduction in a Neotropical rain forest bird. Ecology 81:2458–2472
Wolda H (1978) Seasonal fluctuations in rainfall, food and abundance of tropical insects. J Anim Ecol 47:369–381
Wolda H (1988) Insect seasonality: why? Ann Rev Ecol Syst 19:1–18
Acknowledgments
I thank Martha Robbins and ITFC for generously providing access to leaf phenology data from the Max Planck Gorilla Food Plant Study and the Extended Phenology Study, respectively. I also thank Alastair McNeilage, Miriam van Heist and Douglas Sheil for their hospitality and support, and Derek Pomeroy and Chris Perrins, who initiated the study and encouraged me to take part. Will Cresswell kindly commented on a draft of the manuscript. I also thank Barabara Helm, who made many insightful comments during the review process. Narsensius Owoyesigire, Savio Ngabirano, Margaret Kobusingye, Lawrence Tumugabirwe and David Ebbutt provided valuable assistance in the field. Robert Barigyira advised on the names and status of tree species at Ruhija. I gratefully acknowledge financial support received through the British Ornithologists’ Union Small Research Grant scheme and from the African Bird Club. Uganda Wildlife Authority and the Uganda National Council for Science and Technology granted permission for the study to take place. The field methods used in this study complied fully with the relevant laws applicable in Uganda.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Bairlein.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Shaw, P. Rainfall, leafing phenology and sunrise time as potential Zeitgeber for the bimodal, dry season laying pattern of an African rain forest tit (Parus fasciiventer). J Ornithol 158, 263–275 (2017). https://doi.org/10.1007/s10336-016-1395-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-016-1395-6