Abstract
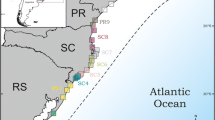
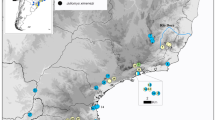
Studies of Atlantic forest (AF) organisms suggest that the historical dynamics of the forest cover produced demographically stable populations in its central region and unstable populations in the southern regions. We studied the mitochondrial phylogeographic structure of an AF passerine, the Greenish Schiffornis Schiffornis virescens (Tityridae), and evaluated questions related to the history of the AF. We analyzed cytochrome b and control region sequences of the mitochondrial genome by traditional phylogenetic and population genetic methods based on summary statistics. In addition, we used coalescent simulations to evaluate specific models of evolution of the populations of S. virescens. The results did not support phylogeographic partitions of the genetic variability of S. virescens. The overall Φst was = 0.32 and gene flow between regions was moderate to high. The analyses suggested that the total population of S. virescens suffered a bottleneck followed by a demographic expansion in the late Pleistocene. The bottleneck might have contributed to the extinction of intraspecific lineages, and hence to the observed lack of a strong phylogeographic pattern and low genetic diversity. Our results suggest that some AF taxa have had all their populations similarly affected by the recent history of the biome, contrary to what has been revealed from most of the other phylogeographic studies in the region and as suggested by a model of AF refuges (the Carnaval–Moritz model). We suggest that the response of organisms to common histories may be idiosyncratic, and predictions about the history of the biome should take into account ecological characteristics and distribution of each specific taxa.
Zusammenfassung
Matrilineare Hinweise auf demografische Expansion, geringe genetische Diversität und fehlende phylogeografische Struktur bei der Olivtrauerkotinga Schiffornis virescens (Aves: Tityridae), einer endemischen Vogelart der Mata Atlantica
Untersuchungen an Organismen der Mata Atlantica deuten darauf hin, dass die historische Dynamik der Waldbedeckung dort demografisch stabile Populationen in den inneren Bereichen und instabile Populationen in den südlichen Regionen hervorgebracht hat. Wir betrachteten die mitochondriale phylogeografische Struktur bei der Olivtrauerkotinga S. virescens (Tityridae), einem Sperlingsvogel der Mata Atlantica, und untersuchten Fragen im Zusammenhang mit der Geschichte dieses atlantischen Waldes. Wir analysierten Sequenzen von Cytochrom b und von Kontrollregionen des mitochondrialen Genoms unter Anwendung der gebräuchlichen phylogenetischen und populationsgenetischen Methoden auf der Grundlage statistischer Parameter. Zusätzlich verwendeten wir Coalescent Simulations, um spezifische Evolutionsmodelle für die Populationen von S. virescens zu testen. Die Ergebnisse konnten keine phylogeografischen Untereinheiten in der genetischen Variabilität von S. virescens belegen. Der Gesamtwert für Φst betrug 0,32 und der Genfluss zwischen den Regionen war mäßig bis hoch. Die Analysen deuten an, dass die gesamte Population von S. virescens zunächst durch einen genetischen Flaschenhals ging, worauf anschließend im Oberen Pleistozän eine demografische Expansion erfolgte. Dieser Flaschenhals könnte zum Aussterben intraspezifischer Abstammungslinien und somit zum beobachteten Mangel an deutlichen phylogeografischen Mustern und zu der geringen genetischen Diversität beigetragen haben. Unsere Ergebnisse legen nahe, dass bei manchen Taxa der Mata Atlantica alle Populationen durch die jüngere Geschichte dieses Bioms ähnlich beeinflusst wurden. Dies steht im Gegensatz zu den Erkenntnissen der meisten anderen phylogeografischen Studien aus dieser Region und zu einem Modell atlantischer Waldrefugien (Carnaval–Moritz-Modell). Wir vermuten, dass verschiedene Organismen selbst auf eine gemeinsame Geschichte in ihrer ganz eigenen Weise reagieren. Prognosen über die Geschichte des Bioms sollten daher immer auch die ökologischen Eigenschaften und die Verbreitung jedes einzelnen Taxons berücksichtigen.



Similar content being viewed by others
References
Aleixo A (2002) Molecular systematics and the role of the “varzea” - “terra-firme” ecotone in the diversification of Xiphorhynchus woodcreepers (Aves : Dendrocolaptidae). Auk 119(3):621–640
Aleixo A (2004) Historical diversification of a Terra-firme forest bird superspecies: a phylogeographic perspective on the role of different hypotheses of Amazonian diversification. Evolution 58(6):1303–1317
Álvarez-Presas M, Carbayo F, Rozas J, Riutort M (2011) Land planarians (Platyhelminthes) as a model organism for fine-scale phylogeographic studies: understanding patterns of biodiversity in the Brazilian Atlantic Forest hotspot. J Evol Biol 24(4):887–896
Amaro RC, Carnaval AC, Yonenaga-Yassuda Y, Trefaut Rodrigues M (2012) Demographic processes in the montane Atlantic rainforest: molecular and Cytogenetic evidence from the endemic frog Proceratophrys boiei. Mol Phylogenet Evol 63:880–888
Anderson CNK, Ramakrishnan U, Chan YL, Hadly EA (2005) Serial SimCoal: a population genetics model for data from multiple populations and points in time. Bioinformatics 21(8):1733–1734
Anderson D, Goudie A, Parker A, Goudie A (2007) Global environments through the Quaternary: exploring environmental change. Oxford University Press, Oxford
Anjos L (2006) Bird Species Sensitivity in a Fragmented Landscape of the Atlantic Forest in Southern Brazil1. Biotropica 38(2):229–234
Anjos L, Collins CD, Holt RD, Volpato GH, Mendonça LB, Lopes EV, Boçon R, Bisheimer MV, Serafini PP, Carvalho J (2011) Bird species abundance-occupancy patterns and sensitivity to forest fragmentation: implications for conservation in the Brazilian Atlantic forest. Biol Conserv 144:2213–2222
Avise JC (2000) Phylogeography: the history and formation of species. Harvard University Press, Cambridge
Baker AJ, Marshall HD (1997) Mitochondrial control region sequences as tools for understanding evolution. In: Mindell DP (ed) Avian molecular evolution and systematics. Academic, San Diego, pp 51–82
Batalha-Filho H, Waldschmidt AM, Campos LAO, Tavares MG, Fernandes-Salomão TM (2010) Phylogeography and historical demography of the Neotropical stingless bee Melipona quadrifasciata (Hymenoptera, Apidae): incongruence between morphology and mitochondrial DNA. Apidologie 41(5):534–547
Batalha-Filho H, Cabanne GS, Miyaki CY (2012) Phylogeography of an Atlantic forest passerine reveals demographic stability through the last glacial maximum. Mol Phylogenet Evol. http://dx.doi.org/10.1016/j.ympev.2012.08.010
Bates JM (2002) The genetic effects of forest fragmentation on five species of Amazonian birds. J Avian Biol 33(3):276–294
Behling H (2002) South and southeast Brazilian grasslands during Late Quaternary times: a synthesis. Palaeogeogr Palaeoclimatol Palaeoecol 177(1–2):19–27
Behling H, Negrelle RRB (2001) Tropical rain forest and climate dynamics of the Atlantic lowland, Southern Brazil, during the late Quaternary. Quat Res 56(3):383–389
Behling H, Arz HW, Patzold J, Wefer G (2002) Late Quaternary vegetational and climate dynamics in southeastern Brazil, inferences from marine cores GeoB 3229–2 and GeoB 3202–1. Palaeogeogr Palaeoclimatol Palaeoecol 179(3–4):227–243
Belle EM, Ramakrishnan U, Mountain JL, Barbujani G (2006) Serial coalescent simulations suggest a weak genealogical relationship between Etruscans and modern Tuscans. Proc Natl Acad Sci USA 103(21):8012–8017
Burney CW, Brumfield RT (2009) Ecology predicts levels of genetic differentiation in Neotropical birds. Am Nat 174(3):358–368
Cabanne GS, Santos FR, Miyaki CY (2007) Phylogeography of Xiphorhynchus fuscus (Passeriformes, Dendrocolaptidae): vicariance and recent demographic expansion in southern Atlantic forest. Biol J Linn Soc 91(1):73–84
Cabanne GS, d’Horta FM, Sari EHR, Santos FR, Miyaki CY (2008) Nuclear and mitochondrial phylogeography of the Atlantic forest endemic Xiphorhynchus fuscus (Aves: Dendrocolaptidae): biogeography and systematics implications. Mol Phylogenet Evol 49:760–773
Cabanne GS, D’Horta FM, Meyer D, Silva JMC, Miyaki CY (2011) Evolution of Dendrocolaptes platyrostris (Aves: Furnariidae) between the South American open vegetation corridor and the Atlantic forest. Biol J Linn Soc 103(4):801–820
Carnaval AC, Moritz C (2008) Historical climate modeling predicts patterns of current biodiversity in the Brazilian Atlantic forest. J Biogeogr 35:1187–1201
Carnaval AC, Hickerson MJ, Haddad CFB, Rodrigues MT, Moritz C (2009) Stability predicts genetic diversity in the Brazilian Atlantic forest hotspot. Science 323(5915):785–789
Chan YL, Anderson CNK, Hadly EA (2006) Bayesian estimation of the timing and severity of a population bottleneck from ancient DNA. PLoS Genet 2(4):451–460
Cheviron ZA, Hackett SJ, Capparella AP (2005) Complex evolutionary history of a Neotropical lowland forest bird (Lepidothrix coronata) and its implications for historical hypotheses of the origin of Neotropical avian diversity. Mol Phylogenet Evol 36(2):338–357
d′Horta FM (2009) Filogenia molecular e filogeografia de passeriformes (Aves): historia biogeográfica da região Neotropical com ênfase na Floresta Atlântica. PhD Thesis. Universidade de São Paulo, São Paulo
d’Horta F, Cabanne GS, Meyer D, Miyaki CY (2011) The genetic effects of Late Quaternary climatic changes over a tropical latitudinal gradient: diversification of an Atlantic Forest passerine. Mol Ecol 20:1923–1935
Drummond A, Rambaut A (2007) BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol 7:214
Excoffier L, Smouse PE, Quattro JM (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes—application to human mitochondrial-DNA restriction data. Genetics 131(2):479–491
Excoffier L, Laval G, Schneider S (2006) Arlequin ver. 3.1. an integrated software package for population genetics data analysis. Computational and molecular population genetics Lab (CMPG), Inst. of Zoology, Univ. of Berne
Fabre V, Condemi S, Degioanni A (2009) Genetic evidence of geographical groups among Neanderthals. PLoS ONE 4(4):e5151
Fitzpatrick SW, Brasileiro CA, Haddad CFB, Zamudio KR (2009) Geographical variation in genetic structure of an Atlantic Coastal Forest frog reveals regional differences in habitat stability. Mol Ecol 18(13):2877–2896
Fu YX (1997) Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 147(2):915–925
Galindo Leal C, Câmara I de G (2003) The Atlantic forest of South America: biodiversity status, threats, and outlook. State of the Hotspots. Island Press, Washington
Grazziotin FG, Monzel M, Echeverrigaray S, Bonatto SL (2006) Phylogeography of the Bothrops jararaca complex (Serpentes: Viperidae): past fragmentation and island colonization in the Brazilian Atlantic Forest. Mol Ecol 15(13):3969–3982
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52(5):696–704
Hedrick PW (2000) Genetics of populations, 2nd edn. Jones and Bartlett, Boston
Hein J, Schierup MH, Wiuf C (2005) Gene genealogies, variation and evolution : a primer in coalescent theory. Oxford University Press, Oxford
Hewitt G (2000) The genetic legacy of the Quaternary ice ages. Nature 405(6789):907–913
Hewitt GM (2004) Genetic consequences of climatic oscillations in the Quaternary. Philos Trans R Soc Lond B 359(1442):183–195
Hey J, Nielsen R (2004) Multilocus methods for estimating population sizes, migration rates and divergence time, with applications to the divergence of Drosophila pseudoobscura and D. persimilis. Genetics 167(2):747–760
Irestedt M, Fjeldsa J, Ericson PGP (2004) Phylogenetic relationships of woodcreepers (Aves: Dendrocolaptinae) incongruence between molecular and morphological data. J Avian Biol 35(3):280–288
Klicka J, Zink RM (1999) Pleistocene effects on North American songbird evolution. Proc R Soc Lond B 266:695–700
Knowles LL (2009) Statistical phylogeography. Annu Rev Ecol Evol Syst 40:593–612
Kuhner MK (2006) LAMARC 2.0: maximum likelihood and Bayesian estimation of population parameters. Bioinformatics 22(6):768–770
Lacerda DR, Marini MA, Santos FR (2007) Mitochondrial DNA corroborates the species distinctiveness of the Planalto (Thamnophilus pelzelni Hellmayr, 1924) and the Sooretama (T. ambiguus Swainson, 1825) Slaty-antshrikes (Passeriformes: Thamnophilidae). Braz J Biol 67:873–882
Ledru MP, Rousseau DD, Cruz FW, Riccomini C, Karmann I, Martin L (2005) Paleoclimate changes during the last 100,000 yr from a record in the Brazilian Atlantic rainforest region and interhemispheric comparison. Quat Res 64(3):444–450
Lemmon AR, Brown JM, Stanger-Hall K, Lemmon EM (2009) The effect of ambiguous data on phylogenetic estimates obtained by maximum likelihood and Bayesian inference. Syst Biol 58(1):130–145
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25(11):1451–1452
Maldonado-Coelho M (2012) Climatic oscillations shape the phylogeographical structure of Atlantic Forest fire-eye antbirds (Aves: Thamnophilidae). Biol J Linn Soc 105:900–924
Martins FM, Gifalli-Iughetti C, Koiffman C, Harris E (2011) Coalescent analysis of mtDNA indicates Pleistocene divergence among three species of howler monkey (Alouatta spp.) and population subdivision within the Atlantic Coastal Forest species, A. guariba. Primates 52(1):77–87
Mata H, Fontana CS, Maurício GN, Bornschein MR, de Vasconcelos MF, Bonatto SL (2009) Molecular phylogeny and biogeography of the eastern Tapaculos (Aves: Rhinocryptidae: Scytalopus, Eleoscytalopus): cryptic diversification in Brazilian Atlantic Forest. Mol Phylogenet Evol 53(2):450–462
Minin VN, Bloomquist EW, Suchard MA (2008) Smooth skyride through a rough skyline: Bayesian coalescent-based inference of population dynamics. Mol Biol Evol 25(7):1459–1471
Mustrangi MA, Patton JL (1997) Phylogeography and systematics of the Slender Mouse Opossum Marmosops (Marsupialia: Didelphidae). University of California Publications in Zoology 130
Nielsen R (2005) Molecular signatures of natural selection. Annu Rev Genet 39(1):197–218
Nielsen R, Beaumont MA (2009) Statistical inferences in phylogeography. Mol Ecol 18(6):1034–1047
Nielsen R, Wakeley J (2001) Distinguishing migration from isolation: a Markov chain Monte Carlo approach. Genetics 158(2):885–896
Nyari AS (2007) Phylogeographic patterns, molecular and vocal differentiation, and species limits in Schiffornis turdina (Aves). Mol Phylogenet Evol 44(1):154–164
Pessoa RO (2007) Sistemática e Biogeografia Histórica da Família Conopophagidae (Aves: Passeriformes): Especiação nas Florestas da América do Sul. Universidade de São Paulo, São Paulo
Pessoa RO, Cabanne GS, Sari EH, Santos FR, Miyaki CY (2006) Comparative phylogeography of the Rufous Gnateater (Conopophagidae) and Lesser Woodcreeper (Dendrocolaptidae): congruent history of two passerines from the south American Atlantic forest. J Ornithol 147(5):227–228
Peter BM, Wegmann D, Excoffier L (2010) Distinguishing between population bottleneck and population subdivision by a Bayesian model choice procedure. Mol Ecol 19(21):4648–4660
Porto TJ, Carnaval AC, da Rocha PLB (2012) Evaluating forest refugial models using species distribution models, model filling and inclusion: a case study with 14 Brazilian species. Divers Distrib. doi:10.1111/j.1472-4642.2012.00944.x
Posada D, Crandall KA (1998) MODELTEST: testing the model of DNA substitution. Bioinformatics 14(9):817–818
Remsen JV, Cadena CD, Jaramillo A, Nores M, Pacheco JF, Robbins MB, Schulenberg TS, Stiles FG, Stotz DF, Zimmer KJ (2009) A classification of the bird species of South America. American Ornithologists’ Union. http://www.museum.lsu.edu/~Remsen/SACCBaseline.html. Accessed 29 Jan 2009
Ribas CC, Gaban-Lima R, Miyaki CY, Cracraft J (2005) Historical biogeography and diversification within the Neotropical parrot genus Pionopsitta (Aves: Psittacidae). J Biogeogr 32(8):1409–1427
Ribon R, Simon JE, De Mattos GT (2003) Bird extinctions in Atlantic forest fragments of the Viçosa region, southeastern Brazil. Conserv Biol 17(6):1827–1839
Richards CL, Carstens BC, Knowles LL (2007) Distribution modelling and statistical phylogeography: an integrative framework for generating and testing alternative biogeographical hypotheses. J Biogeogr 34(11):1833–1845
Ricklefs RE (2007) Estimating diversification rates from phylogenetic information. Trends Ecol Evol 22(11):601–610
Ridgely RS, Tudor G (1996) The birds of South America: the Suboscine Passerines, 1st edn. University of Texas Press, Austin
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19(12):1572–1574
Ruegg KC, Hijmans RJ, Moritz C (2006) Climate change and the origin of migratory pathways in the Swainson’s thrush, Catharus ustulatus. J Biogeogr 33(7):1172–1182
Salisbury CL, Seddon N, Cooney CR, Tobias JA (2012) The latitudinal gradient in dispersal constraints: ecological specialization drives diversification in tropical birds. Ecol Lett 15:847–855
Stotz DF, Fitzpatrick JW, Parker TA III, Moskovits DK (1996) Neotropical birds: ecology and conservation. University of Chicago Press, Chicago
Tajima F (1989) Statistical-method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123(3):585–595
Thomé MTC, Zamudio KR, Giovanelli JGR, Haddad CFB, Baldiserra FA, Alexandrino J (2010) Phylogeography of endemic toads and post-Pliocene persistence of the Brazilian Atlantic Forest. Mol Phylogenet Evol 55:1018–1031
Voight BF, Adams AM, Frisse LA, Qian Y, Hudson RR, Di Rienzo A (2005) Interrogating multiple aspects of variation in a full resequencing data set to infer human population size changes. Proc Natl Acad Sci USA 102(51):18508–18513
Wang JL (2004) Application of the one-migrant-per-generation rule to conservation and management. Conserv Biol 18(2):332–343
Weir JT, Schluter D (2008) Calibrating the avian molecular clock. Mol Ecol 17(10):2321–2328
Whitney BM, Vasconcelos MF, Silveira LF, Pacheco JF (2010) Scytalopus petrophilus (Rock Tapaculo): a new species from Minas Gerais, Brazil. Rev Bras Ornitol 18:73–88
Zeng K, Shi S, Wut CI (2007) Compound tests for the detection of hitchhiking under positive selection. Mol Biol Evol 24(8):1898–1908
Acknowledgments
We thank R.G. Lima, A. Martensen, M. Marini, A. Uezu and J.P. Metzger for providing us with some tissue samples. We also thank IBAMA, Instituto Florestal (SP, Brazil), Instituto Estadual de Florestas (MG, Brazil) and the Ministerio de Ecología de Misiones (Argentina) for the appropriate permits. We are also grateful to Conservación Argentina (G. Zurita and D. Varela), J. Albuquerque, F. d′Horta, R. Pessoa, F. Nodari and T. Matsumoto for assistance during fieldwork. V.A. Ellis, L. Calderon and L. Campagna provided useful comments that improved an early version of the manuscript. Finally, we thank J. Fjeldså and the Associate Editor F. Bairlein for the revision and comments that helped to improve the manuscript. This study was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Brazil), Consejo Nacional de Investigaciones Científicas y Técnicas (Argentina, grant PIP 276), Agencia Nacional de Promoción Científica y Tecnológica (Argentina, grant PICT 805), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (Brazil), Fundação de Amparo à Pesquisa do Estado de São Paulo (Brazil) and WWF (USA). All experiments comply with the current laws of the country in which they were performed. The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Fjeldså.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cabanne, G.S., Sari, E.H.R., Meyer, D. et al. Matrilineal evidence for demographic expansion, low diversity and lack of phylogeographic structure in the Atlantic forest endemic Greenish Schiffornis Schiffornis virescens (Aves: Tityridae). J Ornithol 154, 371–384 (2013). https://doi.org/10.1007/s10336-012-0901-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-012-0901-8