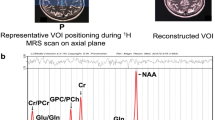
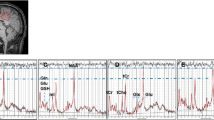
Abstract
Objective
To investigate metabolic changes of mild cognitive impairment in Parkinson’s disease (PD-MCI) using proton magnetic resonance spectroscopic imaging (1H-MRSI).
Methods
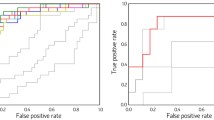
Sixteen healthy controls (HC), 26 cognitively normal Parkinson’s disease (PD-CN) patients, and 34 PD-MCI patients were scanned in this prospective study. Neuropsychological tests were performed, and three-dimensional 1H-MRSI was obtained at 3 T. Metabolic parameters and neuropsychological test scores were compared between PD-MCI, PD-CN, and HC. The correlations between neuropsychological test scores and metabolic intensities were also assessed. Supervised machine learning algorithms were applied to classify HC, PD-CN, and PD-MCI groups based on metabolite levels.
Results
PD-MCI had a lower corrected total N-acetylaspartate over total creatine ratio (tNAA/tCr) in the right precentral gyrus, corresponding to the sensorimotor network (p = 0.01), and a lower tNAA over myoinositol ratio (tNAA/mI) at a part of the default mode network, corresponding to the retrosplenial cortex (p = 0.04) than PD-CN. The HC and PD-MCI patients were classified with an accuracy of 86.4% (sensitivity = 72.7% and specificity = 81.8%) using bagged trees.
Conclusion
1H-MRSI revealed metabolic changes in the default mode, ventral attention/salience, and sensorimotor networks of PD-MCI patients, which could be summarized mainly as ‘posterior cortical metabolic changes’ related with cognitive dysfunction.




Similar content being viewed by others
Availability of data and material (data transparency)
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, Marshall FJ, Ravina BM, Schifitto G, Siderowf A, Tanner CM (2007) Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 68:384–386
Angel RW, Alston W, Higgins JR (1970) Control of movement in Parkinson’s disease. Brain 93:1–14
Schapira AHV, Chaudhuri KR, Jenner P (2017) Non-motor features of Parkinson disease. Nat Rev Neurosci 18:509
Litvan I, Aarsland D, Adler CH, Goldman JG, Kulisevsky J, Mollenhauer B, Rodriguez-Oroz MC, Troster AI, Weintraub D (2011) MDS task force on mild cognitive impairment in Parkinson’s disease: critical review of PD-MCI. Mov Disord 26:1814–1824
Aarsland D, Kurz MW (2010) The epidemiology of dementia associated with Parkinson’s disease. Brain Pathol 20:633–639
Janvin CC, Larsen JP, Aarsland D, Hugdahl K (2006) Subtypes of mild cognitive impairment in Parkinson’s disease: progression to dementia. Mov Disord 21:1343–1349
Chang L, Munsaka SM, Kraft-Terry S, Ernst T (2013) Magnetic resonance spectroscopy to assess neuroinflammation and neuropathic pain. J Neuroimmune Pharmacol 8:576–593
Govindaraju V, Young K, Maudsley AA (2000) Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed 13:129–153
Miller BL (1991) A review of chemical issues in 1H NMR spectroscopy: N-acetyl-l-aspartate, creatine and choline. NMR Biomed 4:47–52
Nie K, Zhang Y, Huang B, Wang L, Zhao J, Huang Z, Gan R, Wang L (2013) Marked N-acetylaspartate and choline metabolite changes in Parkinson’s disease patients with mild cognitive impairment. Park Relat Disord 19:329–334
Camicioli RM, Korzan JR, Foster SL, Fisher NJ, Emery DJ, Bastos AC, Hanstock CC (2004) Posterior cingulate metabolic changes occur in Parkinson’s disease patients without dementia. Neurosci Lett 354:177–180
Hu MT, Taylor-Robinson SD, Chaudhuri KR, Bell JD, Morris RG, Clough C, Brooks DJ, Turjanski N (1999) Evidence for cortical dysfunction in clinically non-demented patients with Parkinson’s disease: a proton MR spectroscopy study. J Neurol Neurosurg Psychiatry 67:20–26
Summerfield C, Gomez-Anson B, Tolosa E, Mercader JM, Marti MJ, Pastor P, Junque C (2002) Dementia in Parkinson disease: a proton magnetic resonance spectroscopy study. Arch Neurol 59:1415–1420
Pagonabarraga J, Gomez-Anson B, Rotger R, Llebaria G, Garcia-Sanchez C, Pascual-Sedano B, Gironell A, Delfino M, Ruscalleda J, Kulisevsky J (2012) Spectroscopic changes associated with mild cognitive impairment and dementia in Parkinson’s disease. Dement Geriatr Cogn Disord 34:312–318
Waragai M, Moriya M, Nojo T (2017) Decreased N-acetyl aspartate/myo-inositol ratio in the posterior cingulate cortex shown by magnetic resonance spectroscopy may be one of the risk markers of preclinical Alzheimer’s disease: a 7-year follow-up study. J Alzheimers Dis 60:1411–1427
Siger M, Schuff N, Zhu X, Miller BL, Weiner MW (2009) Regional myo-inositol concentration in mild cognitive impairment using 1H magnetic resonance spectroscopic imaging. Alzheimer Dis Assoc Disord 23:57–62
Gao F, Barker PB (2014) Various MRS application tools for Alzheimer disease and mild cognitive impairment. AJNR Am J Neuroradiol 35:S4-11
Lang S, Hanganu A, Gan LS, Kibreab M, Auclair-Ouellet N, Alrazi T, Ramezani M, Cheetham J, Hammer T, Kathol I, Sarna J, Monchi O (2019) Network basis of the dysexecutive and posterior cortical cognitive profiles in Parkinson’s disease. Mov Disord 34:893–902
Ahmed RM, Devenney EM, Irish M, Ittner A, Naismith S, Ittner LM, Rohrer JD, Halliday GM, Eisen A, Hodges JR, Kiernan MC (2016) Neuronal network disintegration: common pathways linking neurodegenerative diseases. J Neurol Neurosurg Psychiatry 87:1234–1241
Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, Fischl B, Liu H, Buckner RL (2011) The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106:1125–1165
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Litvan I, Goldman JG, Troster AI, Schmand BA, Weintraub D, Petersen RC, Mollenhauer B, Adler CH, Marder K, Williams-Gray CH, Aarsland D, Kulisevsky J, Rodriguez-Oroz MC, Burn DJ, Barker RA, Emre M (2012) Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: movement disorder society task force guidelines. Mov Disord 27:349–356
Uysal-Canturk P, Hanagasi HA, Bilgic B, Gurvit H, Emre M (2018) An assessment of Movement Disorder Society Task Force diagnostic criteria for mild cognitive impairment in Parkinson’s disease. Eur J Neurol 25:148–153
Shader RI, Harmatz JS, Salzman C (1974) A new scale for clinical assessment in geriatric populations: sandoz clinical assessment-geriatric (SCAG). J Am Geriatr Soc 22:107–113
Akbostanci MC, Bayram E, Yilmaz V, Rzayev S, Ozkan S, Tokcaer AB, Saka E, Durmaz Celik FN, Barut BO, Tufekcioglu Z, Acarer A, Balaban H, Erer S, Dogu O, Kibaroglu S, Aydin N, Hanagasi H, Elibol B, Emre M, Stebbins GT, Goetz CG (2018) Turkish standardization of movement disorders society unified Parkinson’s disease rating scale and unified dyskinesia rating scale. Mov Disord Clin Pr 5:54–59
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Mioshi E, Dawson K, Mitchell J, Arnold R, Hodges JR (2006) The Addenbrooke’s cognitive examination revised (ACE-R): a brief cognitive test battery for dementia screening. Int J Geriatr Psychiatry 21:1078–1085
Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53:695–699
Stroop JR (1935) Studies of interference in serial verbal reactions. J Exp Psychol 18:643–662
Benton AL, Hamsher KD, Varney NR, Spreen O (1983) Judgment of line orientation. Oxford University Press, New York
Smith A (1968) The symbol-digit modalities test: a neuropsychologic test of learning and other cerebral disorders. J Helmuth (Ed), Learn Disord Spec Child Publ Seattle 83–91.
Fey ET (1951) The performance of young schizophrenics and young normals on the Wisconsin card sorting test. J Consult Psychol 15:311–319
Provencher SW (1993) Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 30:672–679
Cengiz S, Yildiri M, Bas A, Ozturk-Isik E (2022) ORYX-MRSI: a fully-automated open-source software for proton magnetic resonance spectroscopic imaging data analysis. Int J Imaging Syst Technol. https://doi.org/10.1002/ima.22748
Smith SM (2002) Fast robust automated brain extraction. Hum Brain Mapp 17:143–155
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23(Suppl 1):S208–S219
Ozturk-Isik E, Crane JC, Cha S, Chang SM, Berger MS, Nelson SJ (2006) Unaliasing lipid contamination for MR spectroscopic imaging of gliomas at 3T using sensitivity encoding (SENSE). Magn Reson Med 55:1164–1169
Doelken MT, Mennecke A, Stadlbauer A, Kecskeméti L, Kasper BS, Struffert T, Doerfler A, Stefan H, Hammen T (2010) Multi-voxel magnetic resonance spectroscopy at 3 T in patients with idiopathic generalised epilepsy. Seizure 19:485–492
Hess AT, Jacobson SW, Jacobson JL, Molteno CD, Van Der Kouwe AJW, Meintjes EM (2014) A comparison of spectral quality in magnetic resonance spectroscopy data acquired with and without a novel EPI-navigated PRESS sequence in school-aged children with fetal alcohol spectrum disorders. Metab Brain Dis 29:323–332
Ozturk-Isik E, Cengiz S, Ozcan A, Yakicier C, Ersen Danyeli A, Pamir MN, Özduman K, Dincer A (2020) Identification of IDH and TERTp mutation status using 1H-MRS in 112 hemispheric diffuse gliomas. J Magn Reson Imaging 51:1799–1809
Jenkinson M, Bannister P, Brady JM, Smith SM (2002) Improved optimisation for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17(2):825–841
Schaefer A, Kong R, Gordon EM, Laumann TO, Zuo XN, Holmes AJ, Eickhoff SB, Yeo BTT (2018) Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb Cortex 28:3095–3114
Kamagata K, Motoi Y, Hori M, Suzuki M, Nakanishi A, Shimoji K, Kyougoku S, Kuwatsuru R, Sasai K, Abe O, Mizuno Y, Aoki S, Hattori N (2011) Posterior hypoperfusion in Parkinson’s disease with and without dementia measured with arterial spin labeling MRI. J Magn Reson Imaging 33:803–807
Syrimi ZJ, Vojtisek L, Eliasova I, Viskova J, Svatkova A, Vanicek J, Rektorova I (2017) Arterial spin labelling detects posterior cortical hypoperfusion in non-demented patients with Parkinson’s disease. J Neural Transm 124:551–557
Arslan DB, Gurvit H, Genc O, Kicik A, Eryurek K, Cengiz S, Erdogdu E, Yildirim Z, Tufekcioglu Z, Ulug AM, Bilgic B, Hanagasi H, Tuzun E, Demiralp T, Ozturk-Isik E (2020) The cerebral blood flow deficits in Parkinson’s disease with mild cognitive impairment using arterial spin labeling MRI. J Neural Transm 127:1285–1294
Azamat S, Betul Arslan D, Erdogdu E, Kicik A, Cengiz S, Eryürek K, Tufekcioglu Z, Bilgic B, Hanagasi H, Demiralp T, Gurvit H, Ozturk-Isik E (2021) Detection of visual and frontoparietal network perfusion deficits in Parkinson’s disease dementia. Eur J Radiol 144:109985
Guan J, Rong Y, Wen Y, Wu H, Qin H, Zhang Q, Chen W (2017) Detection and application of neurochemical profile by multiple regional (1)H-MRS in Parkinson’s disease. Brain Behav 7:e00792
Almuqbel M, Melzer TR, Myall DJ, MacAskill MR, Pitcher TL, Livingston L, Wood KL, Keenan RJ, Dalrymple-Alford JC, Anderson TJ (2016) Metabolite ratios in the posterior cingulate cortex do not track cognitive decline in Parkinson’s disease in a clinical setting. Park Relat Disord 22:54–61
Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007) Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349
Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE (2007) Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA 104:11073
Anderson DE, Ghate DA, Rizzo M (2021) Vision, attention, and driving. Handb Clin Neurol 178:337–360
Uddin LQ (2016) Salience network of the human brain. Salience Netw Hum Brain. https://doi.org/10.1016/C2015-0-01862-7
Norman LJ, Taylor SF, Liu Y, Radua J, Chye Y, De Wit SJ, Huyser C, Karahanoglu FI, Luks T, Manoach D, Mathews C, Rubia K, Suo C, van den Heuvel OA, Yücel M, Fitzgerald K (2019) Error-processing and inhibitory control in obsessive-compulsive disorder: a meta-analysis using statistical parametric maps. Biol Psychiatry 85:713
van Eimeren T, Pellecchia G, Cilia R, Ballanger B, Steeves TD, Houle S, Miyasaki JM, Zurowski M, Lang AE, Strafella AP (2010) Drug-induced deactivation of inhibitory networks predicts pathological gambling in PD. Neurology 75:1711–1716
Buckner RL, Andrews-Hanna JR, Schacter DL (2008) The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38
Raichle ME (2015) The brain’s default mode network. Annu Rev Neurosci 38:433–447
Li W, Xu J, Xiang Q, Zhuo K, Zhang Y, Liu D, Li Y (2022) Neurometabolic and functional changes of default-mode network relate to clinical recovery in first-episode psychosis patients: a longitudinal 1H-MRS and fMRI study. NeuroImage Clin 34:102970
Voevodskaya O, Sundgren PC, Strandberg O, Zetterberg H, Minthon L, Blennow K, Wahlund LO, Westman E, Hansson O (2016) Myo-inositol changes precede amyloid pathology and relate to APOE genotype in Alzheimer disease. Neurology 86:1754–1761
Oeltzschner G, Wijtenburg SA, Mikkelsen M, Edden RAE, Barker PB, Joo JH, Leoutsakos JS, Rowland LM, Workman CI, Smith GS (2019) Neurometabolites and associations with cognitive deficits in mild cognitive impairment: a magnetic resonance spectroscopy study at 7 Tesla. Neurobiol Aging 73:211–218
Diez-Cirarda M, Strafella AP, Kim J, Pena J, Ojeda N, Cabrera-Zubizarreta A, Ibarretxe-Bilbao N (2018) Dynamic functional connectivity in Parkinson’s disease patients with mild cognitive impairment and normal cognition. Neuroimage Clin 17:847–855
Yau Y, Zeighami Y, Baker TE, Larcher K, Vainik U, Dadar M, Fonov VS, Hagmann P, Griffa A, Misic B, Collins DL, Dagher A (2018) Network connectivity determines cortical thinning in early Parkinson’s disease progression. Nat Commun 9:12
Wolpert DH (2018) The relationship between PAC, the statistical physics framework, the Bayesian framework, and the VC framework. Math Gen 117–214.
Cox SR, Dickie DA, Ritchie SJ, Karama S, Pattie A, Royle NA, Corley J, Aribisala BS, Hernandez MV, Maniega SM, Starr JM, Bastin ME, Evans AC, Wardlaw JM, Deary IJ (2016) Associations between education and brain structure at age 73 years, adjusted for age 11 IQ. Neurology 87:1820–1826
Park YW, Deelchand DK, Joers JM, Hanna B, Berrington A, Gillen JS, Kantarci K, Soher BJ, Barker PB, Park H, Oz G, Lenglet C (2018) AutoVOI: real-time automatic prescription of volume-of-interest for single voxel spectroscopy. Magn Reson Med 80:1787–1798
Funding
This study was funded by The Scientific and Technological Research Council of Turkey (TUBITAK) 1001 Grant #115S219 and Istanbul University Scientific Research Projects Unit project #1567/42362.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the study. The recruitment and neuropsychological and clinical assessments of the subjects were conducted by HG, HH, BB, and ZT. The genetic data analysis was performed by AK and ET. MR scanning protocol optimization and data collection were performed by SC, DBA, EE, AK, AMU, TD, and EO-I. New data analysis tools were developed by SC, MY, GHH, and EO-I. Data analysis was performed by SC and DBA. All authors contributed to the interpretation and discussion of the results. The first draft of the manuscript was written by SC and all authors revised it critically for important intellectual content. All authors read and approved the final manuscript. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cengiz, S., Arslan, D.B., Kicik, A. et al. Identification of metabolic correlates of mild cognitive impairment in Parkinson's disease using magnetic resonance spectroscopic imaging and machine learning. Magn Reson Mater Phy 35, 997–1008 (2022). https://doi.org/10.1007/s10334-022-01030-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-022-01030-6