Abstract
Objectives
Innovative physiologic MRI development focuses on depiction of heterogenous vascular and metabolic features in glioblastoma. For this feasibility study, we employed blood oxygenation level-dependent (BOLD) MRI with standardized and precise carbon dioxide (CO2) and oxygen (O2) modulation to investigate specific tumor tissue response patterns in patients with newly diagnosed glioblastoma.
Materials and methods
Seven newly diagnosed untreated patients with suspected glioblastoma were prospectively included to undergo a BOLD study with combined CO2 and O2 standardized protocol. %BOLD signal change/mmHg during hypercapnic, hypoxic, and hyperoxic stimulus was calculated in the whole brain, tumor lesion and segmented volumes of interest (VOI) [contrast-enhancing (CE) − tumor, necrosis and edema] to analyze their tissue response patterns.
Results
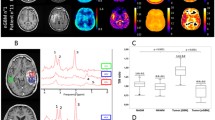
Quantification of BOLD signal change after gas challenges can be used to identify specific responses to standardized stimuli in glioblastoma patients. Integration of this approach with automatic VOI segmentation grants improved characterization of tumor subzones and edema. Magnitude of BOLD signal change during the 3 stimuli can be visualized at voxel precision through color-coded maps overlayed onto whole brain and identified VOIs.
Conclusions
Our preliminary investigation shows good feasibility of BOLD with standardized and precise CO2 and O2 modulation as an emerging physiologic imaging technique to detail specific glioblastoma characteristics. The unique tissue response patterns generated can be further investigated to better detail glioblastoma lesions and gauge treatment response.





Similar content being viewed by others
References
Weller M, van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, Bendszus M, Balana C, Chinot O, Dirven L, French P, Hegi ME, Jakola AS, Platten M, Roth P, Rudà R, Short S, Smits M, Taphoorn MJB, von Deimling A, Westphal M, Soffietti R, Reifenberger G, Wick W (2020) EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. https://doi.org/10.1038/s41571-020-00447-z
Wen PY, Weller M, Lee EQ, Alexander BM, Barnholtz-Sloan JS, Barthel FP, Batchelor TT, Bindra RS, Chang SM, Chiocca EA, Cloughesy TF, DeGroot JF, Galanis E, Gilbert MR, Hegi ME, Horbinski C, Huang RY, Lassman AB, Le Rhun E, Lim M, Mehta MP, Mellinghoff IK, Minniti G, Nathanson D, Platten M, Preusser M, Roth P, Sanson M, Schiff D, Short SC, Taphoorn MJB, Tonn J-C, Tsang J, Verhaak RGW, von Deimling A, Wick W, Zadeh G, Reardon DA, Aldape KD, van den Bent MJ (2020) Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol 22:1073–1113
Lim M, Xia Y, Bettegowda C, Weller M (2018) Current state of immunotherapy for glioblastoma. Nat Rev Clin Oncol 15:422–442
Le Rhun E, Preusser M, Roth P, Reardon DA, van den Bent M, Wen P, Reifenberger G, Weller M (2019) Molecular targeted therapy of glioblastoma. Cancer Treat Rev 80:101896
Ellingson BM (2014) Radiogenomics and imaging phenotypes in glioblastoma: novel observations and correlation with molecular characteristics. Curr Neurol Neurosci Rep 15:506
Mazurowski MA (2015) Radiogenomics: what it is and why it is important. J Am Coll Radiol 12:862–866
Singh G, Manjila S, Sakla N, True A, Wardeh AH, Beig N, Vaysberg A, Matthews J, Prasanna P, Spektor V (2021) Radiomics and radiogenomics in gliomas: a contemporary update. Br J Cancer. https://doi.org/10.1038/s41416-021-01387-w
Clement P, Booth T, Borovečki F, Emblem KE, Figueiredo P, Hirschler L, Jančálek R, Keil VC, Maumet C, Özsunar Y, Pernet C, Petr J, Pinto J, Smits M, Warnert EAH (2021) GliMR: cross-border collaborations to promote advanced MRI biomarkers for glioma. J Med Biol Eng 41:115–125
Hu LS, Hawkins-Daarud A, Wang L, Li J, Swanson KR (2020) Imaging of intratumoral heterogeneity in high-grade glioma. Cancer Lett 477:97–106
Shiroishi MS, Boxerman JL, Pope WB (2015) Physiologic MRI for assessment of response to therapy and prognosis in glioblastoma. Neuro Oncol 18:467–478
Hyare H, Thust S, Rees J (2017) Advanced MRI techniques in the monitoring of treatment of gliomas. Curr Treat Options Neurol 19:11
Boxerman JL, Quarles CC, Hu LS, Erickson BJ, Gerstner ER, Smits M, Kaufmann TJ, Barboriak DP, Huang RH, Wick W, Weller M, Galanis E, Kalpathy-Cramer J, Shankar L, Jacobs P, Chung C, van den Bent MJ, Chang S, Al Yung WK, Cloughesy TF, Wen PY, Gilbert MR, Rosen BR, Ellingson BM, Schmainda KM, Committee JBTDDCISS, Arons DF, Kingston A, Sandak D, Wallace M, Musella A, Haynes C (2020) Consensus recommendations for a dynamic susceptibility contrast MRI protocol for use in high-grade gliomas. Neuro Oncol 22:1262–1275
Boxerman JL, Shiroishi MS, Ellingson BM, Pope WB (2016) Dynamic susceptibility contrast MR imaging in glioma: review of current clinical practice. Magn Reson Imaging Clin N Am 24:649–670
Zhang J, Liu H, Tong H, Wang S, Yang Y, Liu G, Zhang W (2017) Clinical applications of contrast-enhanced perfusion MRI techniques in gliomas: recent advances and current challenges. Contrast Media Mol Imaging 2017:e7064120
Arevalo-Perez J, Peck KK, Young RJ, Holodny AI, Karimi S, Lyo JK (2015) Dynamic contrast-enhanced perfusion MRI and diffusion-weighted imaging in grading of gliomas. J Neuroimaging 25:792–798
Alsaedi A, Doniselli F, Jäger HR, Panovska-Griffiths J, Rojas-Garcia A, Golay X, Bisdas S (2019) The value of arterial spin labelling in adults glioma grading: systematic review and meta-analysis. Oncotarget 10:1589–1601
Falk Delgado A, De Luca F, van Westen D, Falk Delgado A (2018) Arterial spin labeling MR imaging for differentiation between high- and low-grade glioma—a meta-analysis. Neuro Oncol 20:1450–1461
Togao O, Hiwatashi A, Yamashita K, Kikuchi K, Mizoguchi M, Yoshimoto K, Suzuki SO, Iwaki T, Obara M, Van Cauteren M, Honda H (2016) Differentiation of high-grade and low-grade diffuse gliomas by intravoxel incoherent motion MR imaging. Neuro Oncol 18:132–141
Castellano A, Bailo M, Cicone F, Carideo L, Quartuccio N, Mortini P, Falini A, Cascini GL, Minniti G (2021) Advanced imaging techniques for radiotherapy planning of gliomas. Cancers 13:1063
Ellingson BM, Bendszus M, Boxerman J, Barboriak D, Erickson BJ, Smits M, Nelson SJ, Gerstner E, Alexander B, Goldmacher G, Wick W, Vogelbaum M, Weller M, Galanis E, Kalpathy-Cramer J, Shankar L, Jacobs P, Pope WB, Yang D, Chung C, Knopp MV, Cha S, van den Bent MJ, Chang S, Al Yung WK, Cloughesy TF, Wen PY, Gilbert MR, the Jumpstarting Brain Tumor Drug Development Coalition Imaging Standardization Steering Committee, Whitney A, Sandak D, Musella A, Haynes C, Wallace M, Arons DF, Kingston A, Sul J, Krainak D, the Jumpstarting Brain Tumor Drug Development Coalition Imaging Standardization Steering Committee (2015) Consensus recommendations for a standardized Brain Tumor Imaging Protocol in clinical trials. Neuro Oncol 17:1188–1198
Verburg N, Koopman T, Yaqub MM, Hoekstra OS, Lammertsma AA, Barkhof F, Pouwels PJW, Reijneveld JC, Heimans JJ, Rozemuller AJM, Bruynzeel AME, Lagerwaard F, Vandertop WP, Boellaard R, Wesseling P, de Witt Hamer PC (2020) Improved detection of diffuse glioma infiltration with imaging combinations: a diagnostic accuracy study. Neuro Oncol 22:412–422
Suh CH, Kim HS, Jung SC, Choi CG, Kim SJ (2018) 2-Hydroxyglutarate MR spectroscopy for prediction of isocitrate dehydrogenase mutant glioma: a systemic review and meta-analysis using individual patient data. Neuro Oncol 20:1573–1583
Paech D, Windschuh J, Oberhollenzer J, Dreher C, Sahm F, Meissner J-E, Goerke S, Schuenke P, Zaiss M, Regnery S, Bickelhaupt S, Bäumer P, Bendszus M, Wick W, Unterberg A, Bachert P, Ladd ME, Schlemmer H-P, Radbruch A (2018) Assessing the predictability of IDH mutation and MGMT methylation status in glioma patients using relaxation-compensated multipool CEST MRI at 7.0 T. Neuro Oncol 20:1661–1671
Englander ZK, Horenstein CI, Bowden SG, Chow DS, Otten ML, Lignelli A, Bruce JN, Canoll P, Grinband J (2018) Extent of BOLD vascular dysregulation is greater in diffuse gliomas without isocitrate dehydrogenase 1 R132H mutation. Radiology 287:965–972
Stadlbauer A, Zimmermann M, Heinz G, Oberndorfer S, Doerfler A, Buchfelder M, Rössler K (2017) Magnetic resonance imaging biomarkers for clinical routine assessment of microvascular architecture in glioma. J Cereb Blood Flow Metab 37:632–643
Stadlbauer A, Zimmermann M, Doerfler A, Oberndorfer S, Buchfelder M, Coras R, Kitzwögerer M, Roessler K (2018) Intratumoral heterogeneity of oxygen metabolism and neovascularization uncovers 2 survival-relevant subgroups of IDH1 wild-type glioblastoma. Neuro Oncol 20:1536–1546
Goerke S, Soehngen Y, Deshmane A, Zaiss M, Breitling J, Boyd PS, Herz K, Zimmermann F, Klika KD, Schlemmer H-P, Paech D, Ladd ME, Bachert P (2019) Relaxation-compensated APT and rNOE CEST-MRI of human brain tumors at 3 T. Magn Reson Med 82:622–632
Leao DJ, Craig PG, Godoy LF, Leite CC, Policeni B (2020) Response assessment in neuro-oncology criteria for gliomas: practical approach using conventional and advanced techniques. Am J Neuroradiol 41:10–20
Ellingson BM, Wen PY, Cloughesy TF (2018) Evidence and context of use for contrast enhancement as a surrogate of disease burden and treatment response in malignant glioma. Neuro Oncol 20:457–471
Sebök M, van Niftrik CHB, Muscas G, Pangalu A, Seystahl K, Weller M, Regli L, Fierstra J (2021) Hypermetabolism and impaired cerebrovascular reactivity beyond the standard MRI-identified tumor border indicate diffuse glioma extended tissue infiltration. Neuro Oncol Adv. https://doi.org/10.1093/noajnl/vdab048
Shaikh F, Dupont-Roettger D, Dehmeshki J, Awan O, Kubassova O, Bisdas S (2020) The role of imaging biomarkers derived from advanced imaging and radiomics in the management of brain tumors. Front Oncol. https://doi.org/10.3389/fonc.2020.559946
Strauss SB, Meng A, Ebani EJ, Chiang GC (2019) Imaging glioblastoma posttreatment: progression, pseudoprogression, pseudoresponse, radiation necrosis. Radiol Clin North Am 57:1199–1216
Zikou A, Sioka C, Alexiou GA, Fotopoulos A, Voulgaris S, Argyropoulou MI (2018) Radiation necrosis, pseudoprogression, pseudoresponse, and tumor recurrence: imaging challenges for the evaluation of treated gliomas. Contrast Media Mol Imaging 2018:e6828396
Muscas G, van Niftrik CHB, Sebök M, Della Puppa A, Seystahl K, Andratschke N, Brown M, Weller M, Regli L, Piccirelli M, Fierstra J (2021) Distinct cerebrovascular reactivity patterns for brain radiation necrosis. Cancers 13:1840
Slessarev M, Han J, Mardimae A, Prisman E, Preiss D, Volgyesi G, Ansel C, Duffin J, Fisher JA (2007) Prospective targeting and control of end-tidal CO2 and O2 concentrations. J Physiol 581:1207–1219
Fierstra J, van Niftrik C, Piccirelli M, Bozinov O, Pangalu A, Krayenbühl N, Valavanis A, Weller M, Regli L (2018) Diffuse gliomas exhibit whole brain impaired cerebrovascular reactivity. Magn Reson Imaging 45:78–83
Muscas G, van Niftrik CHB, Sebök M, Seystahl K, Piccirelli M, Stippich C, Weller M, Regli L, Fierstra J (2020) Hemodynamic investigation of peritumoral impaired blood oxygenation-level dependent cerebrovascular reactivity in patients with diffuse glioma. Magn Reson Imaging 70:50–56
Fierstra J, van Niftrik B, Piccirelli M, Burkhardt JK, Pangalu A, Kocian R, Valavanis A, Weller M, Regli L, Bozinov O (2016) Altered intraoperative cerebrovascular reactivity in brain areas of high-grade glioma recurrence. Magn Reson Imaging 34:803–808
Attwell D, Buchan AM, Charpak S, Lauritzen M, MacVicar BA, Newman EA (2010) Glial and neuronal control of brain blood flow. Nature 468:232–243
Watkins S, Robel S, Kimbrough IF, Robert SM, Ellis-Davies G, Sontheimer H (2014) Disruption of astrocyte–vascular coupling and the blood–brain barrier by invading glioma cells. Nat Commun 5:4196
Montgomery MK, Kim SH, Dovas A, Zhao HT, Goldberg AR, Xu W, Yagielski AJ, Cambareri MK, Patel KB, Mela A, Humala N, Thibodeaux DN, Shaik MA, Ma Y, Grinband J, Chow DS, Schevon C, Canoll P, Hillman EMC (2020) Glioma-induced alterations in neuronal activity and neurovascular coupling during disease progression. Cell Rep 31:107500
Bowden SG, Gill BJA, Englander ZK, Horenstein CI, Zanazzi G, Chang PD, Samanamud J, Lignelli A, Bruce JN, Canoll P, Grinband J (2018) Local glioma cells are associated with vascular dysregulation. AJNR Am J Neuroradiol 39:507–514
Iranmahboob A, Peck KK, Brennan NP, Karimi S, Fisicaro R, Hou B, Holodny AI (2016) Vascular reactivity maps in patients with gliomas using breath-holding BOLD fMRI. J Neuroimaging 26:232–239
Hsu Y-Y, Chang C-N, Jung S-M, Lim K-E, Huang J-C, Fang S-Y, Liu H-L (2004) Blood oxygenation level-dependent MRI of cerebral gliomas during breath holding. J Magn Reson Imaging 19:160–167
Holodny AI, Schulder M, Liu WC, Maldjian JA, Kalnin AJ (1999) Decreased BOLD functional MR activation of the motor and sensory cortices adjacent to a glioblastoma multiforme: implications for image-guided neurosurgery. Am J Neuroradiol 20:609–612
Holodny AI, Schulder M, Liu W-C, Wolko J, Maldjian JA, Kalnin AJ (2000) The effect of brain tumors on BOLD functional MR imaging activation in the adjacent motor cortex: implications for image-guided neurosurgery. Am J Neuroradiol 21:1415–1422
Corroyer-Dulmont A, Chakhoyan A, Collet S, Durand L, MacKenzie ET, Petit E, Bernaudin M, Touzani O, Valable S (2015) Imaging modalities to assess oxygen status in glioblastoma. Front Med. https://doi.org/10.3389/fmed.2015.00057
Gérard M, Corroyer-Dulmont A, Lesueur P, Collet S, Chérel M, Bourgeois M, Stefan D, Limkin EJ, Perrio C, Guillamo J-S, Dubray B, Bernaudin M, Thariat J, Valable S (2019) Hypoxia imaging and adaptive radiotherapy: a state-of-the-art approach in the management of glioma. Front Med. https://doi.org/10.3389/fmed.2019.00117
Virani N, Kwon J, Zhou H, Mason R, Berbeco R, Protti A (2021) In vivo hypoxia characterization using blood oxygen level dependent magnetic resonance imaging in a preclinical glioblastoma mouse model. Magn Reson Imaging 76:52–60
Christen T, Schmiedeskamp H, Straka M, Bammer R, Zaharchuk G (2012) Measuring brain oxygenation in humans using a multiparametric quantitative blood oxygenation level dependent MRI approach. Magn Reson Med 68:905–911
Christen T, Lemasson B, Pannetier N, Farion R, Remy C, Zaharchuk G, Barbier EL (2012) Is T2* enough to assess oxygenation? Quantitative blood oxygen level-dependent analysis in brain tumor. Radiology 262:495–502
Chédeville AL, Madureira PA (2021) The role of hypoxia in glioblastoma radiotherapy resistance. Cancers 13:542
Colwell N, Larion M, Giles AJ, Seldomridge AN, Sizdahkhani S, Gilbert MR, Park DM (2017) Hypoxia in the glioblastoma microenvironment: shaping the phenotype of cancer stem-like cells. Neuro Oncol 19:887–896
Mendichovszky I, Jackson A (2011) Imaging hypoxia in gliomas. BJR 84:S145–S158
Bekaert L, Valable S, Lechapt-Zalcman E, Ponte K, Collet S, Constans J-M, Levallet G, Bordji K, Petit E, Branger P, Emery E, Manrique A, Barré L, Bernaudin M, Guillamo J-S (2017) [18F]-FMISO PET study of hypoxia in gliomas before surgery: correlation with molecular markers of hypoxia and angiogenesis. Eur J Nucl Med Mol Imaging 44:1383–1392
Preibisch C, Shi K, Kluge A, Lukas M, Wiestler B, Göttler J, Gempt J, Ringel F, Jaberi MA, Schlegel J, Meyer B, Zimmer C, Pyka T, Förster S (2017) Characterizing hypoxia in human glioma: a simultaneous multimodal MRI and PET study. NMR Biomed 30:e3775
Stadlbauer A, Kinfe TM, Eyüpoglu I, Zimmermann M, Kitzwögerer M, Podar K, Buchfelder M, Heinz G, Oberndorfer S, Marhold F (2020) Tissue hypoxia and alterations in microvascular architecture predict glioblastoma recurrence in humans. Clin Cancer Res. https://doi.org/10.1158/1078-0432.CCR-20-3580
Stadlbauer A, Kinfe TM, Zimmermann M, Eyüpoglu I, Brandner N, Buchfelder M, Zaiss M, Dörfler A, Brandner S (2020) Association between tissue hypoxia, perfusion restrictions, and microvascular architecture alterations with lesion-induced impairment of neurovascular coupling. J Cereb Blood Flow Metab. https://doi.org/10.1177/0271678X20947546
Vu C, Chai Y, Coloigner J, Nederveen AJ, Borzage M, Bush A, Wood JC (2021) Quantitative perfusion mapping with induced transient hypoxia using BOLD MRI. Magn Reson Med 85:168–181
MacDonald ME, Berman AJL, Mazerolle EL, Williams RJ, Pike GB (2018) Modeling hyperoxia-induced BOLD signal dynamics to estimate cerebral blood flow, volume and mean transit time. Neuroimage 178:461–474
Stieb S, Riesterer O, Boss A, Weiss T, Guckenberger M, Özbay PS, Nanz D, Rossi C (2019) Dependency of the blood oxygen level dependent-response to hyperoxic challenges on the order of gas administration in intracranial malignancies. Neuroradiology 61:783–793
Özbay PS, Stieb S, Rossi C, Riesterer O, Boss A, Weiss T, Kuhn FP, Pruessmann KP, Nanz D (2018) Lesion magnetic susceptibility response to hyperoxic challenge: a biomarker for malignant brain tumor microenvironment? Magn Reson Imaging 47:147–153
Bashat DB, Artzi M, Ami HB, Aizenstein O, Blumenthal DT, Bokstein F, Corn BW, Ram Z, Kanner AA, Lifschitz-Mercer B, Solar I, Kolatt T, Palmon M, Edrei Y, Abramovitch R (2012) Hemodynamic response imaging: a potential tool for the assessment of angiogenesis in brain tumors. PLoS ONE 7:e49416
Laufer S, Mazuz A, Nachmansson N, Fellig Y, Corn BW, Bokstein F, Bashat DB, Abramovitch R (2014) Monitoring brain tumor vascular heamodynamic following anti-angiogenic therapy with advanced magnetic resonance imaging in mice. PLoS ONE 9:e115093
Sobczyk O, Fierstra J, Venkatraghavan L, Poublanc J, Duffin J, Fisher JA, Mikulis DJ (2021) Measuring cerebrovascular reactivity: sixteen avoidable pitfalls. Front Physiol 12:990
Duffin J, Sobczyk O, Crawley AP, Poublanc J, Mikulis DJ, Fisher JA (2015) The dynamics of cerebrovascular reactivity shown with transfer function analysis. Neuroimage 114:207–216
van Niftrik CHB, Piccirelli M, Bozinov O, Pangalu A, Fisher JA, Valavanis A, Luft AR, Weller M, Regli L, Fierstra J (2017) Iterative analysis of cerebrovascular reactivity dynamic response by temporal decomposition. Brain Behav 7:e00705
Kassner A, Winter JD, Poublanc J, Mikulis DJ, Crawley AP (2010) Blood-oxygen level dependent MRI measures of cerebrovascular reactivity using a controlled respiratory challenge: reproducibility and gender differences. J Magn Reson Imaging 31:298–304
Fisher JA, Lashmi V, Mikulis DJ (2018) Magnetic resonance imaging-based cerebrovascular reactivity and hemodynamic reserve. Stroke 49:2011–2018
Delgado-López PD, Riñones-Mena E, Corrales-García EM (2018) Treatment-related changes in glioblastoma: a review on the controversies in response assessment criteria and the concepts of true progression, pseudoprogression, pseudoresponse and radionecrosis. Clin Transl Oncol 20:939–953
Lasocki A, Gaillard F (2019) Non-contrast-enhancing tumor: a new frontier in glioblastoma research. Am J Neuroradiol 40:758–765
Karschnia P, Vogelbaum MA, van den Bent M, Cahill DP, Bello L, Narita Y, Berger MS, Weller M, Tonn J-C (2021) Evidence-based recommendations on categories for extent of resection in diffuse glioma. Eur J Cancer 149:23–33
Niyazi M, Brada M, Chalmers AJ, Combs SE, Erridge SC, Fiorentino A, Grosu AL, Lagerwaard FJ, Minniti G, Mirimanoff R-O, Ricardi U, Short SC, Weber DC, Belka C (2016) ESTRO-ACROP guideline “target delineation of glioblastomas.” Radiother Oncol 118:35–42
Horská A, Barker PB (2010) Imaging of brain tumors: MR spectroscopy and metabolic imaging. Neuroimaging Clin N Am 20:293–310
Hakyemez B, Erdogan C, Bolca N, Yildirim N, Gokalp G, Parlak M (2006) Evaluation of different cerebral mass lesions by perfusion-weighted MR imaging. J Magn Reson Imaging 24:817–824
Di N, Cheng W, Chen H, Zhai F, Liu Y, Mu X, Chu Z, Lu N, Liu X, Wang B (2019) Utility of arterial spin labelling MRI for discriminating atypical high-grade glioma from primary central nervous system lymphoma. Clin Radiol 74:165.e1-165.e9
Xi Y, Kang X, Wang N, Liu T, Zhu Y, Cheng G, Wang K, Li C, Guo F, Yin H (2019) Differentiation of primary central nervous system lymphoma from high-grade glioma and brain metastasis using arterial spin labeling and dynamic contrast-enhanced magnetic resonance imaging. Eur J Radiol 112:59–64
Soni N, Srindharan K, Kumar S, Mishra P, Bathla G, Kalita J, Behari S (2018) Arterial spin labeling perfusion: prospective MR imaging in differentiating neoplastic from non-neoplastic intra-axial brain lesions. Neuroradiol J 31:544–553
Shah AH, Snelling B, Bregy A, Patel PR, Tememe D, Bhatia R, Sklar E, Komotar RJ (2013) Discriminating radiation necrosis from tumor progression in gliomas: a systematic review what is the best imaging modality? J Neurooncol 112:141–152
Wu H, Tong H, Du X, Guo H, Ma Q, Zhang Y, Zhou X, Liu H, Wang S, Fang J, Zhang W (2020) Vascular habitat analysis based on dynamic susceptibility contrast perfusion MRI predicts IDH mutation status and prognosis in high-grade gliomas. Eur Radiol 30:3254–3265
Pang H, Dang X, Ren Y, Zhuang D, Qiu T, Chen H, Zhang J, Ma N, Li G, Zhang J, Wu J, Feng X (2019) 3D-ASL perfusion correlates with VEGF expression and overall survival in glioma patients: comparison of quantitative perfusion and pathology on accurate spatial location-matched basis. J Magn Reson Imaging 50:209–220
Law M, Young RJ, Babb JS, Peccerelli N, Chheang S, Gruber ML, Miller DC, Golfinos JG, Zagzag D, Johnson G (2008) Gliomas: predicting time to progression or survival with cerebral blood volume measurements at dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 247:490–498
Seeger A, Braun C, Skardelly M, Paulsen F, Schittenhelm J, Ernemann U, Bisdas S (2013) Comparison of three different MR perfusion techniques and MR spectroscopy for multiparametric assessment in distinguishing recurrent high-grade gliomas from stable disease. Acad Radiol 20:1557–1565
Dou W, Lin C-YE, Ding H, Shen Y, Dou C, Qian L, Wen B, Wu B (2019) Chemical exchange saturation transfer magnetic resonance imaging and its main and potential applications in pre-clinical and clinical studies. Quant Imaging Med Surg 9:1747766–1741766
Morrison MA, Lupo JM (2021) 7-T magnetic resonance imaging in the management of brain tumors. Magn Reson Imaging Clin N Am 29:83–102
Baudelet C (2005) Current issues in the utility of blood oxygen level dependent MRI for the assessment of modulations in tumor oxygenation. Curr Med Imaging 1:229–243
Losert C, Peller M, Schneider P, Reiser M (2002) Oxygen-enhanced MRI of the brain. Magn Reson Med 48:271–277
Fierstra J, Sobczyk O, Battisti-Charbonney A, Mandell DM, Poublanc J, Crawley AP, Mikulis DJ, Duffin J, Fisher JA (2013) Measuring cerebrovascular reactivity: what stimulus to use? J Physiol 591:5809–5821
Sebök M, van Niftrik CHB, Halter M, Hiller A, Seystahl K, Pangalu A, Weller M, Stippich C, Regli L, Fierstra J (2020) Crossed cerebellar diaschisis in patients with diffuse glioma is associated with impaired supratentorial cerebrovascular reactivity and worse clinical outcome. Cerebellum 19:824–832
Sobczyk O, Battisti-Charbonney A, Fierstra J, Mandell DM, Poublanc J, Crawley AP, Mikulis DJ, Duffin J, Fisher JA (2014) A conceptual model for CO2-induced redistribution of cerebral blood flow with experimental confirmation using BOLD MRI. Neuroimage 92:56–68
Lüdemann L, Förschler A, Grieger W, Zimmer C (2006) BOLD signal in the motor cortex shows a correlation with the blood volume of brain tumors. J Magn Reson Imaging 23:435–443
Johnston AJ, Steiner LA, Gupta AK, Menon DK (2003) Cerebral oxygen vasoreactivity and cerebral tissue oxygen reactivity† †AJJ is supported by an unrestricted neurosciences intensive care research grant from Codman. LAS is supported by grants from the Margarete und Walter Lichtenstein-Stiftung (Basel, Switzerland), a Myron B. Laver Grant (Department of Anaesthesia, University of Basel, Switzerland) and the Swiss National Science Foundation, and is recipient of an Overseas Research Student Award (Committee of Vice-Chancellors and Principals of the Universities of the United Kingdom). Br J Anaesth 90:774–786
Bickler PE, Feiner JR, Lipnick MS, Batchelder P, MacLeod DB, Severinghaus JW (2017) Effects of acute, profound hypoxia on healthy humans: implications for safety of tests evaluating pulse oximetry or tissue oximetry performance. Anesth Analg 124:146–153
Moreton FC, Dani KA, Goutcher C, O’Hare K, Muir KW (2016) Respiratory challenge MRI: practical aspects. NeuroImage Clin 11:667–677
Schwarzbauer C, Deichmann R (2012) Vascular component analysis of hyperoxic and hypercapnic BOLD contrast. Neuroimage 59:2401–2412
Song Y, Cho G, Chun S-I, Baek JH, Cho H, Kim YR, Park SB, Kim JK (2014) Oxygen-induced frequency shifts in hyperoxia: a significant component of BOLD signal. NMR Biomed 27:835–842
Ma Y, Berman AJL, Pike GB (2016) The effect of dissolved oxygen on the relaxation rates of blood plasma: Implications for hyperoxia calibrated BOLD. Magn Reson Med 76:1905–1911
Bonekamp D, Mouridsen K, Radbruch A, Kurz FT, Eidel O, Wick A, Schlemmer H-P, Wick W, Bendszus M, Østergaard L, Kickingereder P (2017) Assessment of tumor oxygenation and its impact on treatment response in bevacizumab-treated recurrent glioblastoma. J Cereb Blood Flow Metab 37:485–494
Fisher JA, Mikulis DJ (2021) Cerebrovascular reactivity: purpose, optimizing methods, and limitations to interpretation—a personal 20-year Odyssey of (Re)searching. Front Physiol. https://doi.org/10.3389/fphys.2021.629651
Willie CK, Macleod DB, Shaw AD, Smith KJ, Tzeng YC, Eves ND, Ikeda K, Graham J, Lewis NC, Day TA, Ainslie PN (2012) Regional brain blood flow in man during acute changes in arterial blood gases. J Physiol 590:3261–3275
Willie CK, Tzeng Y-C, Fisher JA, Ainslie PN (2014) Integrative regulation of human brain blood flow. J Physiol 592:841–859
Hoiland RL, Bain AR, Rieger MG, Bailey DM, Ainslie PN (2015) Hypoxemia, oxygen content, and the regulation of cerebral blood flow. Am J Physiol Regul Integr Compar Physiol 310:R398–R413
Maralani PJ, Das S, Mainprize T, Phan N, Bharatha A, Keith J, Munoz DG, Sahgal A, Symons S, Ironside S, Faraji-Dana Z, Eilaghi A, Chan A, Alcaide-Leon P, Shearkhani O, Jakubovic R, Atenafu EG, Zaharchuk G, Mikulis D (2018) Hypoxia detection in infiltrative astrocytoma: ferumoxytol-based quantitative BOLD MRI with intraoperative and histologic validation. Radiology 288:821–829
Funding
This project is funded by the Swiss Cancer League, KFS-3975-08-2016-R.
Author information
Authors and Affiliations
Contributions
Study conception and design (VS, MS, CHBN, JF), Acquisition of data (VS, MS, CHBN, JF), Analysis and interpretation of data (VS, CHBN, MS, JF), Drafting of manuscript (VS, JF), Critical revision (VS, MS, CHBN, KS, NH, ZK, MW, LR, JF).
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical standard
The study was approved by the cantonal ethics board of the Canton of Zurich, Switzerland (research protocol KEK-ZH-No. 2012–0427).
Informed consent
All participants signed informed consent prior to inclusion into the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

10334_2021_980_MOESM2_ESM.tif
Supplementary file2 S2. Individual patient BOLD signal change during hypercapnic (A) and hypoxic and hyperoxic stimulus (B). (TIF 445 KB)

10334_2021_980_MOESM4_ESM.jpg
Supplementary file4 S3. Binarized tumor map analysis workflow. A. Tumor masks are obtained and overlayed onto the hyperoxic, hypoxic and hypercapnic map. B. Only tumor voxels are then considered downstream for binarization analysis. C. For each of the 3 experimental condition (hyperoxia, hypoxia and hypercapnia) the tumor voxels with positive and negative BOLD signal change are identified. D. In positive and negative tumor voxels from each map, the mean %BOLD signal change/mmHg is calculated in the other two experimental conditions. Only the hyperoxic binarized map shows differences between positive versus negative voxels (red, left) (JPG 90 KB)
10334_2021_980_MOESM5_ESM.tif
Supplementary file5 S4. Software-based VOIs segmentation. Left, Input sequences. Right, Segmented masks output (A. Combined segmented masks; B. Edema; C. Contrast enhancing tumor; D. Necrosis) (TIF 316 KB)
Rights and permissions
About this article
Cite this article
Stumpo, V., Sebök, M., van Niftrik, C.H.B. et al. Feasibility of glioblastoma tissue response mapping with physiologic BOLD imaging using precise oxygen and carbon dioxide challenge. Magn Reson Mater Phy 35, 29–44 (2022). https://doi.org/10.1007/s10334-021-00980-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-021-00980-7



