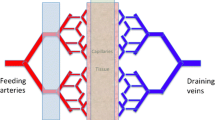
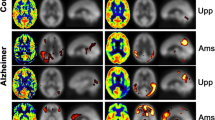
Abstract
Objective
To determine the feasibility of 3D TGSE PASL MRI with long inversion times to estimate CNS perfusion clearance, comparing normals to Alzheimer disease patients.
Methods
This pilot study used 3D TGSE PASL MRI with long TIs to estimate the signal clearance of labeled blood/ultra-filtrate (CSF) from brain signal averages of seven inversion times (TI) from six regions of the brain in 18 normal subjects of ages 18–70 years before and after exercise. Arterial pulse corrected signal average per TI versus TI was plotted. The slope (linear regression) indicated the clearance rate. Three subjects with mild Alzheimer disease (AD) were studied pre-exercise only.
Results
In normals, signal decay rate variance among brain regions, age groups and post-exercise failed to demonstrate statistical significance except in middle-age group pre- to post-exercise-dominant temporal lobe. We found highly statistically significant reduced signal clearance rate in the AD group.
Discussion
Signal decay in normal age groups correlates with decay of T1blood, thus CSF paravascular flow egresses and is inseparable from venous outflow. The AD group correlates with decay rate T1CSF, indicating a proportion of labeled blood ultra-filtered within the brain (paravascular fluid) is retained. This provides indirect evidence of reduced paravascular clearance in AD. Further development may produce an efficient biomarker identifying neurodegenerative diseases and future treatment efficacy.



Similar content being viewed by others
References
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC et al (2015) Structural and functional features of central nervous system lymphatic vessels. Nature. https://doi.org/10.1038/nature14432. (Nature Publishing Group)
Louveau A, Plog B, Antila S, Alitalo K, Nedergaard M, Kipnis J (2017) Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J Clin Invest 127(9):3210–3219. https://doi.org/10.1172/JCI90603
Brinker T, Stopa E, Morrison J, Klinge P (2014) A new look at cerebrospinal fluid circulation. Fluids Barriers CNS 11:10. https://doi.org/10.1186/2045-8118-11-10
Günther M, Oshio K, Feinberg DA (2005) Single-shot 3D imaging techniques improve arterial spin labeling perfusion measurements. Magn Reson Med 54(2):491–498. https://doi.org/10.1002/mrm.20580
Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez-Garcia L, Zaharchuk G et al (2015) Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 73(1):102–116. https://doi.org/10.1002/mrm.25197
MacDonald ME et al (2018) Modeling hyperoxia-induced bold signal dynamics to estimate cerebral blood flow, volume and mean transit time. NeuroImage 178:461–474. https://doi.org/10.1016/J.NEUROIMAGE.2018.05.066. (Academic Press)
Yan L, Liu CY et al (2016) Assessing Intracranial Vascular Compliance Using Dynamic Arterial Spin Labeling. Neuroimage 124(Pt A):433–41. doi:10.1016/j.neuroimage.2015.09.008 (NIH Public Access)
Holter KE, Kehlet B, Devor A et al (2017) Interstitial solute transport in 3D reconstructed neuropil occurs by diffusion rather than bulk flow. Proc Natl Acad Sci USA 114(37):9894–9899. https://doi.org/10.1073/pnas.1706942114
Ohene Y, Harrison IF, Nahavandi P et al (2019) Non-invasive MRI of brain clearance pathways using multiple echo time arterial spin labelling: an aquaporin-4 study. NeuroImage 188:515–523. https://doi.org/10.1016/j.neuroimage.2018.12.026
Fernández-Seara MA, Wang Z, Wang J, Rao H-Y, Guenther M, Feinberg DA, Detre JA (2005) Continuous arterial spin labeling perfusion measurements using single shot 3D GRASE at 3 T. Magn Reson Med. https://doi.org/10.1002/mrm.20674
Feinberg DA, Beckett A, Chen L (2013) Arterial spin labeling with simultaneous multi-slice echo planar imaging. Magn Reson Med 70:1500–1506. https://doi.org/10.1002/mrm.24994
Chao LL, Buckley S, Kornak J, Schuff N, Madison C, Yaffe K, Miller BL (2010) ASL perfusion MRI predicts cognitive decline and conversion from mci to dementia. Alzheimer Dis Assoc Disord 24:19–27. https://doi.org/10.1097/WAD.0b013e3181b4f736
Yan L, Liu CY, Smith RX, Jog M, Langham M, Krasileva K, Wang DJJ (2016) Assessing intracranial vascular compliance using dynamic arterial spin labeling. NeuroImage 124(Pt A):433–441. https://doi.org/10.1016/j.neuroimage.2015.09.008
Smith AJ, Yao X, Dix JA, Jin B, Verkman AS (2017) Test of the “glymphatic” hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. ELife 2017:6. https://doi.org/10.7554/eLife.27679
Abbott J (2004) Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int 45:545–552. https://doi.org/10.1016/j.neuint.2003.11.006
Fisher JP, Hartwich D, Seifert T, Olesen ND, Mcnulty CL, Nielsen HB, Secher NH (2013) Cerebral perfusion, oxygenation and metabolism during exercise in young and elderly individuals. J Physiol 591(7):1859–1870. https://doi.org/10.1113/jphysiol.2012.244905
Tanaka H, Monahan K, Seals D (2001) Age-predicted maximal heart rate revisited. J Am Coll Cardiol 37:153–156. https://www.ncbi.nlm.nih.gov/pubmed/11153730
Lu H, Clingman C, X Golay, MR Peter (2019) Determining the longitudinal relaxation time (T1) of blood at 3.0 Tesla. Wiley Online Library. https://onlinelibrary.wiley.com/doi/abs/10.1002/mrm.20178
Bipin M, Coppo B, Frances S, McGivney D, Ian HJ, Chen Y, Jiang Y, Alan GM et al (2019) Magnetic resonance fingerprinting: a technical review. Magn Reson Med 81(1):25–46. https://doi.org/10.1002/mrm.27403
Ringstad G, Vatnehol S, Eide P (2017) Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain 140:2691–2705. https://doi.org/10.1093/brain/awx191
Lefferts WK, Augustine JA, Heffernan KS (2014) Effect of acute resistance exercise on carotid artery stiffness and cerebral blood flow pulsatility. Front Physiol 5:101. https://doi.org/10.3389/fphys.2014.0010121
Querido JS, Sheel W (2007) Regulation of cerebral blood flow during exercise. Sports Med 37:765–782. https://doi.org/10.2165/00007256-200737090-00002
Ogoh S, Ainslie PN (2009) Cerebral blood flow during exercise: Mechanisms of regulation. J Appl Physiol 107:371380. https://doi.org/10.1152/japplphysiol.00573.2009
Bering E (1952) Water exchange of central nervous system and cerebrospinal fluid. J Neurosurg 9:275–287. https://thejns.org/view/journals/j-neurosurg/9/3/j-neurosurg.9.issue-3.xml
Plog BA, Nedergaard M (2018) The glymphatic system in central nervous system health disease: past, present, and future. Annu Rev Pathol Mech Dis 13(1):379–394. https://doi.org/10.1146/annurev-pathol-051217-111018
Igarshi H, Tsujita M, Kwee IL, Nakada T (2014) Water influx into cerebrospinal fluid is primarily controlled by aquaporin-4, not aquaporin-1. NeuroReport 25:39–43. https://doi.org/10.1097/WNR.0000000000000042
Papadopoulos MC, Verkman AS (2013) Aquaporin water channels in the nervous system. Nature Rev Neurosci 14:265–277. https://doi.org/10.1038/nrn3468
Da Mesquita S, Louveau A, Vaccari A, Smirnov I, Cornelison C, Kingsmore K, Contarino C (2018) Functional aspects of meningeal lymphatics in aging and Alzheimer's disease. Nature 560:185–191. https://doi.org/10.1038/s41586-018-0368-8
Billinger SA, Vidoni ED, Greer CS, Graves RS, Mattlage AE, Burns JM (2014) Cardiopulmonary exercise testing is well tolerated in people with Alzheimer-related cognitive impairment. Arch Phys Med Rehabil 95:1714–1718. https://doi.org/10.1016/j.apmr.2014.04.007
Femminella G, Rengo G, Komici K, Iacotucci P, Petraglia L, Pagano G, De Lucia C (2014) Autonomic dysfunction in Alzheimer's disease: tools for assessment and review of the literature. J Alzheimer's Dis 42:369–377. https://doi.org/10.3233/JAD-140513
Shen Z, Lei J, Li X, Wang Z, Bao X, Wang R (2018) Multifaceted assessment of the APP/PS1 mouse model for Alzheimer’s disease: applying MRS, DTI, and ASL. Brain Res 1698:114–120. https://doi.org/10.1016/j.brainres.2018.08.001
Wolk DA, Detre JA (2012) Arterial spin labeling MRI: an emerging biomarker for Alzheimer’s disease and other neurodegenerative conditions. Curr Opin Neurol 25(4):421–428. https://doi.org/10.1097/WCO.0b013e328354ff0a
Acknowledgements
We would like to thank CENTRA Health Department of Radiology and especially Stan Gray, RPT, for their help and support and in making this project possible. We would like to thank the following medical students at Liberty University College of Osteopathic Medicine (LUCOM) for their enthusiasm and interest and participation in screening our subjects and aiding during the studies: Deanna Pickett, BS, Bridgett Dillon, BS, Kaitlyn Kuntzman, BS, and Tori Diedring. We would also like to thank Julia Sharp, PhD, and Kimberly Love, PhD, for their assistance in the statistical analysis and data analytics. We would also like to thank the LUCOM research committee for their thoughts and recommendations, especially Dr. Kenneth J Dormer, PhD, Anthony J Bauer, PhD, Joseph C Gigliotti, PhD, Joseph W Brewer, PhD, and Jeffery Jaspers, PhD.
Funding
No external funding was received. All funding was from an intramural grant from Liberty University College of Osteopathic Medicine 2017-02.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Charles R Joseph, MD, declares no conflict of interest. Christopher M Benhatzel, BS, declares no conflict of interest. Logan J Stern, BS, declares no conflict of interest. Olivia M Hopper, BS, declares no conflict of interest. Michael D Lockwood, DO, declares no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Joseph, C.R., Benhatzel, C.M., Stern, L.J. et al. Pilot study utilizing MRI 3D TGSE PASL (arterial spin labeling) differentiating clearance rates of labeled protons in the CNS of patients with early Alzheimer disease from normal subjects. Magn Reson Mater Phy 33, 559–568 (2020). https://doi.org/10.1007/s10334-019-00818-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-019-00818-3