Abstract
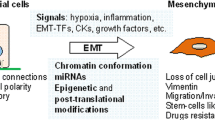
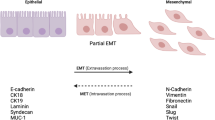
Epithelial-mesenchymal transition (EMT) is initially considered as a physiological phenomenon during the embryogenesis of mammals, as well as a basic biological event maintaining the stability of the vital body. Recent researches indicated that EMT plays a critical role in various tumors progression, through which epithelial cancers invade and metastasize. The cell characteristics are changed during EMT, in which the cells lose cell-cell and cell-matrix interactions and apical polarity, reorganize their cytoskeleton, and become isolated, motile, as well as resistant to anoikis, then become spindle-shaped mesenchymal cells. This review lays emphasis on studying the cell morphogenesis, makers and molecular mechanism regulation about EMT, discussing the relationship between the EMT and the cancer development and metastasis.
Similar content being viewed by others
References
Trimboli AJ, Fukino K, de Bruin A, et al. Direct evidence for epithelial-mesenchymal transitions in breast cancer. Cancer Res, 2008, 68: 937–945.
Gallo D, Ferlini C, Scambia G, et al. The epithelial-mesenchymal transition and theestrogen-signaling in ovarian cancer. Curr Drug Targets, 2010, 11: 474–481.
Brabletz T, Hlubek F, Spaderna S, et al. Invasion and metastasis in colorectal cancer: epithelial-mesenchymaltransition, mesenchymalepithelial transition, stem cells and beta-catenin. Cells Tissues Organs, 2005, 179: 56–65.
Usami Y, Satake S, Nakayama F, et al. Snail-associated epithelial-mesenchymal transition promotes oesophageal squamous cell carcinoma motility and progression. J Pathol, 2008, 215: 330–339.
Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest, 2003, 112: 1776–1784.
Moustakas A, Heldin CH. Signaling networks guiding epithelial-mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci, 2007, 98: 1512–1520.
Valcourt U, Kowanetz M, Niimi H, et al. TGF-beta and the Smad signaling pathway support transcriptomic reprogramming during epithelial-mesenchymal cell transition. Mol Biol Cell, 2005, 16: 1987–2002.
Rajasekaran SA, Huynh TP, Wolle DG, et al. Na, K-ATPase subunits as markers for epithelial-mesenchymal transition in cancer and fibrosis. Mol Cancer Ther, 2010, 9: 1515–1524.
Fuchs BC, Fujii T, Dorfman JD, et al. Epithelial-to-mesenchymal transition and integrin-linked kinase mediate sensitivity to epidermal growth factor receptor inhibition in human hepatoma cells. Cancer Res, 2008, 68: 2391–2399.
Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol, 2001, 2: 285–293.
Zeisberg M, Kalluri R. The role of epithelial-to-mesenchymal transition in renal fibrosis. J Mol Med, 2004, 82: 175–181.
Xie L, Law BK, Chytil AM, et al. Activation of the Erk pathway is required for TGF-β1-induced EMT in vitro. Neoplasia, 2004, 6: 603–610.
Chen L, Liu BC, Zhang XL, et al. Influence of connective tissue growth factor antisense oligonucleotide on angiotensin II-induced epithelial mesenchymal transition in HK2 cells. Acta Pharmacol Sin, 2006, 27: 1029–1036.
Yang J, Liu Y. Dissection of key events in tubular epithelial to myofibroblasttransition and its implications in renal interstitial fifibrosis. Am J Pathol, 2001, 159: 1465–1475.
Barr S, Thomson S, Buck E, et al. Bypassing cellular EGF receptor dependence through epithelial-to-mesenchymal-like transitions. Clin Exp Metastasis, 2008, 25: 685–693.
Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Current Opin Cell Biol, 2005, 17: 548–558.
Nelson WJ, Nusse R. Convergence of Wnt, b-catenin, and cadherin pathways. Science, 2004, 303: 1483–1487.
Peinado H, Portillo F, Cano A. Transcriptional regulation of cadherins during development and carcinogenesis. Int J Dev Biol, 2004, 48: 365–375.
Katoh Y, Katoh M. Hedgehog signaling, epithelial-to-mesenchymal transition and miRNA. Int J Mol Med, 2008, 22: 271–275.
Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol, 2002, 3: 155–166.
Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer, 2007, 7: 415–428.
Kong B, Michalski CW, Hong X, et al. AZGP1 is a tumor suppressor inpancreatic cancer inducing mesenchymal-to-epithelial transdifferentiation by inhibiting TGF-beta-mediated ERK signaling. Oncogene, 2010.
Pantuck AJ, An J, Liu H et al. NF-kappaB-dependent plasticity of the epithelial to mesenchymal transition induced by Von Hippel-Lindau inactivationin renal cell carcinomas. Cancer Res, 2010, 70: 752–761.
Yu M, Smolen GA, Zhang J, et al. A developmentally regulated inducer of EMT, LBX1, contributes to breast cancer progression. Genes Dev, 2009, 23: 1737–1742.
Lee TK, Poon RT, Yuen AP, et al. Twist overexpression correlates with hepatocellular carcinoma metastasis through induction of epithelialmesenchymal transition. Clin Cancer Res, 2006, 12: 5369–5376.
Matsuo N, Shiraha H, Fujikawa T, et al. Twist expression promotes migration and invasion in hepatocellular carcinoma. BMC Cancer, 2009, 9: 240.
Rosivatz E, Becker I, Specht K, et al. Differential expression of the epithelial-mesenchymal transition regulators snail, SIP1, and twist in gastric cancer. Am J Pathol, 2002, 161: 1881–1891.
Cicchini C, Laudadio I, Citarella F, et al. TGFβ-induced EMT requires focal adhesion kinase (FAK) signaling. Exp Cell Res, 2007, 314: 143–152.
Siegel PM, Massagué J. Cytostatic and apoptotic actions of TGF-β in homeostasis and cancer. Nat Rev Cancer, 2003, 3: 807–821.
Tang B, Vu M, Booker T, et al. TGF-beta switches from tumor suppressor to prometastatic factor in a model of breast cancer progression. J Clin Invest, 2003, 112: 1116–1124.
Wendt MK, Allington TM, Schiemann WP. Mechanisms of epithelial- mesenchymal transition by TGF-β. Future Oncol, 2009, 5: 1145–1168.
Lin HY, Wang XF, Ng-Eaton, E, et al. Expression cloning of the TGF-β type II receptor, a functional transmembrane serine/threonine kinase. Cell, 1992, 775-785.
Franzén P, ten Dijke P, Ichijo H, et al. Cloning of a TGFβ type I receptor that forms a heteromeric complex with the TGF β type II receptor. Cell, 1993, 75: 681–692.
Heldin CH, Miyazono K, ten Dijke P. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature, 1997, 390: 465–471.
Massagué J, Seoane J, Wotton D. Smad transcription factors. Genes, 2005, 19: 2783–2810.
Petersen M, Pardali E, van der Horst G, et al. Smad2 and Smad3 have opposing roles in breastcancer bone metastasis by differentially affecting tumor angiogenesis. Oncogene, 2010, 29: 1351–1361.
Moustakas A, Heldin CH. Non-Smad TGF-β signals. J Cell Sci, 2005, 118: 3573–3584.
Galliher AJ, Schiemann WP. Src phosphorylates Tyr284 in TGF-β type II receptor and regulates TGF-β stimulation of p38 MAPK during breast cancer cell proliferation and invasion. Cancer Res, 2007, 67: 3752–3758.
Galliher AJ, Schiemann WP. β3 integrin and Src facilitate TGF-β mediatedinduction of epithelial-mesenchymal transition in mammary epithelial cells. Breast Cancer Res, 2006, 8: R42.
Lamouille S, Derynck R. Cell size and invasion in TGF-β induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. J Cell Biol, 2007, 178: 437–451.
Lee HS, Kim C, Kim SB, et al. Epithin, a target of transforming growth factor-beta signaling, mediates epithelial-mesenchymal transition. Biochem Biophys Res Commun, 2010, 395: 553–559.
MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell, 2009, 17: 9–26.
Nelson WJ and Nusse R. Convergence of Wnt, b-catenin, and cadherin pathways. Science, 2004, 303: 1483–1487.
Blavier L, Lazaryev A, Shi XH, et al. Stromelysin-1 (MMP-3) is a target and a regulator of Wnt1-induced epithelial-mesenchymal transition (EMT). Cancer Biol Ther, 2010, 10: 198–208.
Xu P, Yu SZ, Jiang RC, et al. Differential expression of Notch family members in astrocytomas and medulloblastomas. Pathol Oncol Res, 2009, 15: 703–710.
Fan X, Mikolaenko I, Elhassan I, et al. Notch1 and Notch2 have opposite effects on embryonal brain tumor growth. Cancer Res, 2004, 64: 7787–7793.
Grego-Bessa J, Diez J, Timmerman L, et al. Notch and epithelialmesenchyme transition in development and tumor progression: another turn of the screw. Cell Cycle, 2004, 3: 718–721.
Timmerman LA, Grego-Bessa J, Raya A, et al. Notch promotes epithelial- mesenchymal transition during cardiac developmentand oncogenic transformation. Genes Dev, 2003, 18: 99–115.
Saad S, Stanners SR, Yong R, et al. Notch mediated epithelial to mesenchymal transformation is associated with increased expression of the Snail transcription factor. Int J Biochem Cell Biol, 2010, 42: 1115–1122.
Wang Z, Banerjee S, Li Y, et al. Down-regulation of notch-1 inhibits invasion by inactivation of nuclear factor-kappaB, vascular endothelial growth factor, and matrix metallopro-teinase-9 in pancreatic cancer cells. Cancer Res, 2006, 66: 2778–2784.
Balint K, Xiao M, Pinnix CC, et al. Activation of Notch1 signaling is required for beta-catenin-mediated human primary melanoma progression. J Clin Invest, 2005, 115: 3166–3176.
Pepinsky RB, Rayhorn P, Day ES, et al. Mapping sonic hedgehogreceptor interactions by steric interference. J Biol Chem, 2000, 275: 10995–11001.
Pasca di Magliano M, Hebrok M. Hedgehog signaling in cancer formation andmaintenance. Nat Rev Cancer, 2003, 3: 903–911.
Sanchez P, Hernández AM, Stecca B, et al. Inhibition of prostate cancer proliferation by interference with SONIC HEDGEHOG-GLI1 signaling. Proc Natl Acad Sci USA, 2004, 101: 12561–12566.
Feldmann G, Dhara S, Fendrich V, et al. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: A new paradigm for combination therapy in solid cancers. Cancer Res, 2007, 67: 2187–2196.
Katoh Y, Katoh M. Hedgehog signaling, epithelial-to-mesenchymal transitionand miRNA. Int J Mol Med, 2008, 22: 271–275.
Bailey J, Singh PK, Hollingsworth MA. Cancer metastasis facilitated by developmental pathways: Sonic hedgehog, Notch, and bone morphogenetic proteins. J Cell Biochem, 2007, 102: 829–839.
Katoh M. Networking of WNT, FGF, Notch, BMP, and Hedgehog signalingpathways during carcinogenesis. Stem Cell Rev, 2007, 3: 30–38.
Strippoli R, Benedicto I, Pérez Lozano ML, et al. Epithelial-to-mesenchymal transition of peritoneal mesothelial cells is regulated by an ERK/NF-κB/Snail1 pathway. Dis Model Mech, 2008, 1: 264–274.
Huber MA, Azoitei N, Baumann B, et al. NF-κB is essential for epithelial- mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest, 2004, 114: 569–581.
Birchmeier C, Birchmeier W, Gherardi E, et al. Met, metastasis, motility and more. Nat Rev Mol Cell Biol, 2003, 4: 915–925.
Savagner P, Yamada KM, Thiery JP. The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelial-mesenchymal transition. J Cell Biol, 1997, 137: 1403–1419.
Gregory PA, Bert AG, Paterson EL, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol, 2008, 10: 593–601.
Dyrskjøt L, Ostenfeld MS, Bramsen JB, et al. Genomic profiling of microRNAs in bladdercancer: miR-129 is associated with poor outcome and promotes cell death in vitro. Cancer Res, 2009, 69: 4851–4860.
Burk U, Schubert J, Wellner U, et al. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep, 2008, 9: 582–589.
Bracken CP, Gregory PA, Kolesnikoff N, et al. A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res, 2008, 68: 7846–7854.
Wellner U, Schubert J, Burk UC, et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol, 2009, 11: 1487–1495.
Braun J, Hoang-Vu C, Dralle H, et al. Downregulation of microRNAs directs the EMT and invasive potential of anaplastic thyroid carcinomas. Oncogene, 2010, 29: 4237–4244.
Yu J, Ohuchida K, Mizumoto K, et al. MicroRNA, hsa-miR-200c, is an independent prognostic factor in pancreatic cancer and its upregulation inhibits pancreatic cancer invasion but increases cell proliferation. Mol Cancer, 2010, 9: 169.
Vetter G, Saumet A, Moes M, et al. miR-661 expression in SNAI1-induced epithelial to mesenchymal transition contributes to breast cancer cell invasion by targeting Nectin-1 and StarD10 messengers. Oncogene, 2010, 29: 4436–4448.
Gebeshuber CA, Zatloukal K, Martinez J. miR-29a suppresses tristet- raprolin, which is a regulator of epithelial polarity and metastasis. EMBO Rep, 2009, 10: 400–405.
Nam EJ, Yoon H, Kim SW, et al. MicroRNA expression profiles in serous ovarian carcinoma. Clin Cancer Res, 2008, 14: 2690–2695.
Bonnomet A, Brysse A, Tachsidis A, et al. Epithelial-to-mesenchymal transitions and circulating tumor cells. J Mammary Gland Biol Neoplasia, 2010, 15: 261–273.
Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer, 2002, 2: 442–454.
Moody SE, Perez D, Pan TC, et al. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell, 2005, 8: 197–209.
Aktas B, Tewes M, Fehm T, et al. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res, 2009, 11: R46.
Loric S, Paradis V, Gala JL, et al. Abnormal E-cadherin expression and prostate cell blood dissemination as markers of biological recurrence in cancer. Eur J Cancer, 2001, 37: 1475–1481.
Ray ME, Mehra R, Sandler HM, et al. E-cadherin protein expression predicts prostate cancer salvage radiotherapy outcomes. J Urol, 2006, 176: 1409–1414.
Derycke L, DeWever O, Stove V, et al. Soluble N-cadherin in human biological fluids. Int J Cancer, 2006, 119: 2895–2900.
Jaggi M, Nazemi T, Abrahams NA, et al. N-cadherin switching occurs in high Gleason grade prostate cancer. Prostate, 2006, 66: 193–199.
Chunthapong J, Seftor EA, Khalkhali-Ellis Z, et al. Dual roles of E-cadherin in prostate cancer invasion. J Cell Biochem, 2004, 91: 649–661.
Veveris-Lowe TL, Lawrence MG, Collard RL, et al. Kallikrein 4 (hK4) and prostate-specific antigen (PSA) are associated with the loss of Ecadherin and an epithelial-mesenchymal transition (EMT)-like effect in prostate cancer. Endocr Relat Cancer, 2005, 12: 631–643.
Whitbread AK, Veveris-Lowe TL, Lawrence MG, et al. The role of kallikrein- related peptidases in prostate cancer: Potential involvement in an epithelial to mesenchymal transition. Biol Chem, 2006, 387: 707–714.
Karhadkar SS, Bova GS, Abdallah N, et al. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature, 2004, 431: 707–712.
Xu J, Wang R, Xie ZH, et al. Prostate cancer metastasis: Role of the host microenvironment in promoting epithelial to mesenchymal transition and increased bone and adrenal gland metastasis. Prostate, 2006, 66: 1664–1673.
Shinagawa K, Kitadai Y, Tanaka M, et al. Mesenchymal stem cells enhance growth and metastasis of colon cancer. Int J Cancer, 2010, 127: 2323–2333.
McConkey DJ, Choi W, Marquis L, et al. Role of epithelial-to-mesenchymal transition (EMT) in drug sensitivity and metastasis in bladder cancer. Cancer Metastasis Rev, 2009, 28: 335–344.
Wallerand H, Robert G, Pasticier G, et al. The epithelial-mesenchymal transition-inducing factor TWIST is an attractive target in advanced and/or metastatic bladder and prostate cancers. Urol Oncol, 2010, 28: 473–479.
Yang MH, Chen CL, Chau GY, et al. Comprehensive analysis of the independent effect of twist and snail in promoting metastasis of hepatocellular carcinoma. Hepatology, 2009, 50: 1464–1474.
Lee TK, Poon RT, Yuen AP, et al. Twist over expression correlates with hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin Cancer Res, 2006, 12: 5369–5376.
Wang Y, Xue TC, Xie XY, et al. Relationship between epithelial-mesenchymal transition and lung metastasis in hepatocellular carcinoma. Chin J Surg (Chinese), 2008, 46: 1624–1627.
Hiwatashi K, Ueno S, Sakoda M, et al. Strong Smad4 expression correlates with poor prognosis after surgery in patients with hepatocellular carcinoma. Ann Surg Oncol, 2009, 16: 3176–3182.
Jang TJ, Jeon KH, Jung KH. Cyclooxygenase-2 expression is related to the epithelial-to-mesenchymal transition in human colon cancers. Yonsei Med J, 2009, 50: 818–824.
Zhang W, Jiang B, Guo Z, et al. Four-and-a-half LIM protein 2 promotes invasive potential and epithelial-mesenchymal transition in colon cancer. Carcinogenesis, 2010, 31: 1220–1209.
Varnat F, Duquet A, Malerba M, et al. Human colon cancer epithelial cells harbour active HEDGEHOG-GLI signalling that is essential for tumour growth, recurrence, metastasis and stem cell survival and expansion. EMBO Mol Med, 2009, 1: 338–351.
Jin H, Yu Y, Zhang T, et al. Snail is critical for tumor growth and metastasis of ovarian carcinoma. Int J Cancer, 2010, 126: 2102–2111.
Yeasmin S, Nakayama K, Rahman MT, et al. Loss of MKK4 expression in ovarian cancer. A potential role for the epithelial to mesenchymal transition. Int J Cancer, 2011, 128: 94–104.
Ponnusamy MP, Lakshmanan I, Jain M, et al. MUC4 mucin-induced epithelial to mesenchymal transition: a novel mechanism for metastasis of human ovarian cancer cells. Oncogene, 2010, 29: 6084.
Sasaki K, Natsugoe S, Ishigami S, et al. Significance of Twist expression and its association with E-cadherin in esophageal squamous cell carcinoma. J Exp Clin Cancer Res, 2009, 28: 158.
Cai Z, Wang Q, Zhou Y, et al. Epidermal growth factor-induced epithelial-mesenchymal transition in human esophageal carcinoma cells-a model for the study of metastasis. Cancer Lett, 2010, 296: 88–95.
Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res, 2006, 66: 8319–8326.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the grants from the Natural Science Foundation of China (No. 81000998) and Natural Science Foundation of Hubei Province of China (No. 2007ABA248).
Rights and permissions
About this article
Cite this article
Deng, J., Xu, X. Epithelial-mesenchymal transition and cancermetastasis. Chin. -Ger. J. Clin. Oncol. 10, 125–133 (2011). https://doi.org/10.1007/s10330-011-0740-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10330-011-0740-8