Abstract
Citrus fibrous root tissue was evaluated as an alternative source material for huanglongbing (HLB) diagnosis by real-time PCR using primer-probe set TXCChlb, developed in the present study based on 16S rDNA of “Candidatus Liberibacter asiaticus” (CLas). Real-time PCR data obtained with DNA samples prepared from leaf and fibrous root tissue collected from mature (> 10 years old) and young (4–5 years old) citrus trees growing in Texas and Florida confirmed that root HLB test is more sensitive than the leaf HLB test in terms of the detection rate of HLB positive trees. In addition, the current study confirmed that HLB can be diagnosed at the pre-symptomatic stage using the root HLB test to facilitate efficient removal of HLB-positive, asymptomatic trees that could serve as a source for HLB. The new HLB diagnostic method using root tissue described in the current study can assist in deploying a more efficient disease management strategy to deter HLB spread, especially at the pre-symptomatic stage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Huanglongbing (HLB), also known as citrus greening, is the most serious disease affecting citrus production in Asia, Africa, Americas and the Arabian Peninsula (Bové 2006; Gottwald 2010). HLB has been associated with three related but distinct Gram-negative phloem-inhabiting α-Proteobacteria “Candidatus Liberibacter asiaticus” (CLas) (Jagoueix et al. 1994), “Ca. L. africanus” (CLaf) (Planet et al. 1995), and “Ca. L. americanus” (CLam) (Teixeira et al. 2005). Among these three “Ca. Liberibacter” species, CLas is the most widespread and poses the greatest threat to the citrus industry around the world, while CLaf and CLam are geographically isolated, mostly in Africa and South America, respectively (da Graça and Korsten 2004; Gottwald 2010; Teixeira et al. 2005). In the United States, HLB caused by CLas was first found in Florida in 2005 (Gottwald 2010), and subsequently reported from several states including Georgia, Louisiana, South Carolina, Texas, and California (Wang and Trivedi 2013). Because the pathogen is spread by psyllids (Capoor et al. 1967; McClean and Oberholzer 1965; Wang and Trivedi 2013) and through propagation of infected material, a three-pronged disease management approach that includes inoculum reduction by the removal of trees with HLB disease, control of psyllid vector populations, and production of healthy trees for planting is recommended (da Graça and Korsten 2004).
Pre-symptomatic detection of CLas is critical for the success of inoculum reduction programs because the pathogen can be spread by the vector from newly infected leaves within 15 days after CLas introduction by psyllids and may be a major mode of HLB spread in an orchard (Lee et al. 2015). However, detection of CLas infection is difficult due to the fastidious nature of the bacterium, the uneven distribution of CLas in the citrus tree canopy and the prolonged incubation periods before symptoms are visible—from a few months to 1 or more years after natural inoculation (Bové 2006; Gottwald 2010; Shen et al. 2013). Moreover, in combination, these characteristics can further delay detection of the disease. For example, efficient surveys are hampered by the patchy distribution of the symptoms used to identify trees with sufficient probability of CLas detection. In this situation, delayed symptom development hinders detection (Gottwald et al. 2008; Lee et al. 2015; Li et al. 2006).
Although CLas is introduced into a healthy tree via psyllid feeding on leaves, Johnson et al. (2014) found that root tissues exhibited damage before symptoms became visible on leaves. Moreover, recent studies have shown that CLas distribution is much more uniform in the roots than in the canopy (Louzada et al. 2016). Together, these findings suggest that roots represent a better sampling pool for CLas detection (Kunta et al. 2014a; Louzada et al. 2016).
The most widely used real-time PCR primer-probe set, HLBaspr targeting the CLas 16S rDNA region, however, gives unreliable results for root samples due to nonspecific amplification (Kunta et al. 2014b; Louzada et al. 2016). Such nonspecific amplicons are not produced by other real-time PCR primer-probe sets available for CLas detection in roots such as CQULA (CQULA04F/CQULA04R and TaqMan probe CQULAP10) (Wang et al. 2006), LJ900fpr (Morgan et al. 2012), and RNR (Zheng et al. 2016). The genomic regions targeted by these primer sets are rplJ/rplL ribosomal protein gene for CQULA (Wang et al. 2006), hyvI/hyvII multiple tandem repeats for LJ900fpr (Morgan et al. 2012), and nrdB gene for RNR (Zheng et al. 2016), which are different from that of the HLBaspr primer-probe set (Li et al. 2006).
Bacterial 16S rDNA has been extensively investigated as a tool for the identification and phylogenetic study of prokaryotes due to its evolutionarily conserved nature and the presence of a range of variable regions (Yarza et al. 2010). In the current study, we therefore investigated the nucleotide sequence variation present in 16S rDNAs between CLas and other “Ca. Liberibacter” spp. that are associated with the diseases on citrus and potato to develop a new set of real-time PCR primers for the detection of HLB-causing bacteria from citrus root tissue. The current study developed a new set of primers and probe based on the nucleotide sequence of CLas 16S rDNA and investigated its usefulness for HLB detection in root tissue of pre-symptomatic citrus trees.
Materials and methods
Multiple nucleotide sequence analysis
The 16S rDNA sequences of CLam (NC022793), Ca. L. solanacearum (NC014774), CLaf (NZ_CP004021), CLas (NC012985, NZ_CP010804, NC020549, NZ_AP014595), which had complete genomic sequences available in the NCBI GenBank (https://www.ncbi.nlm.nih.gov) when the current study was initiated, were used for multiple nucleotide sequence alignment using Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo). A new set of primers (TXCChlb-f; 5′-CTTTTCGGAGACCTTTACACA-3′ and TXCChlb-r; 5′-CTTGATGGCAACTAGAGGCA-3′) and a probe (TXCChlb-p; 5′-TGCGCTCGTTGCGGGACTTA-3′) were designed from the region conserved among CLas strains, but not with other “Ca. Liberibacter” species, using Primer3 (Koressaar and Remm 2007) followed by visual inspection (Fig. 1). The amplification efficiency of the newly designed primer-probe set, TXCChlb, was examined by a procedure described below in real-time PCR and conventional PCR (cPCR).
Multiple nucleotide sequence alignment of 16S ribosomal DNA sequence from “Candidatus Liberibacter” spp. GenBank ID and a strain of each Ca. L. americanus (CLam), Ca. L. solanacearum (CLso), Ca. L. africanus (CLaf) and Ca. L. asiaticus (CLas). The sequence of primers (TXCChlb-f and -r) and TaqMan probe (TXCChlb-p) are boxed and in bold italics. Arrows indicate the orientation of primers and probe. Asterisk indicates conserved nucleotide sequence among 16S rDNAs of “Ca. Liberibacter” spp
Fibrous root tissue sampling and DNA extraction
Fibrous roots were collected from 2 to 5 inches below the soil surface at four different quadrants within 2 feet from the tree trunk, which were then pooled and air-dried in a paper bag at room temperature (~ 25 °C) for about 24 h to facilitate easy removal of excessive soil by tapping with fingers. Root DNA was extracted from ~ 150 mg of finely sliced root tissue using a DNeasy PowerPlant Pro HTP 96 kit (Qiagen, Hilden, Germany) and the manufacturer’s instruction with some modification (Supplementary Document 1). PCR inhibitors from the root DNA fraction were removed by using a PCR inhibitor removal kit (Zymo Research, Irvine, CA, USA) followed by 1:1 dilution with sterilized dH2O to minimize potential effects of any remaining PCR inhibitors (Supplementary Document 1). Two and 4 µl of root DNA extracts were used for real-time PCR and cPCR, respectively, following the PCR parameters described below.
Real-time PCR and conventional PCR
The amplification efficiency of the newly developed primer-probe set (TXCChlb) was examined using the OI1–OI2c amplicon (Jagoueix et al. 1996) amplified from CLas 16S rDNA and cloned into pCR2.1 TOPO vector (Invitrogen, Carlsbad, CA, USA). A series of 1:10 dilutions of the plasmid containing the OI1–OI2c amplicon was prepared with sterilized dH2O for use as a template for the real-time PCR for the amplification efficiency test. The 25 µl volume solution for the test contained 6 mM MgCl2, 0.24 mM dNTPs, 240 nM forward and reverse primers, 120 nM TaqMan probe and 1 U Platinum Taq DNA Polymerase (Invitrogen); thermocycling (CFX96 Touch Real-Time PCR Detection System, Hercules, CA, USA) conditions were 1 cycle of 95 °C for 2 min and 40 cycles of 95 °C for 10 s followed by 40 s at 58 °C. These real-time PCR conditions were used throughout the study. Gene copy number and amplification efficiency were calculated using tools available at http://cels.uri.edu/gsc/cndna.html and at the Thermo Fisher Scientific website (https://www.thermofisher.com), respectively.
cPCR was conducted using primer sets OI1/OI2c (Jagoueix et al. 1996) and LSS/Las606 (Fujikawa and Iwanami 2012), which target CLas 16S rDNA. Four microliters of root DNA was used for cPCR in the 50-µl reaction mixture containing 2.5 mM MgCl2, 0.2 mM dNTPs, 200 nM of each forward and reverse primers, OI1 (5′-GCGCGTATGCAATACGAGCGGCA-3′) and OI2c (5′-GCCTCGCGACTTCGCAACCCAT-3′) (Jagoueix et al. 1996), or LSS (5′-ACCCAACATCTAGGTAAAAACC-3′) and Las606 (5′-GGAGAGGTGAGTGGAATTCCG A-3′) (Fujikawa and Iwanami 2012), and 1 U Platinum Taq DNA Polymerase (Invitrogen) in 1× PCR buffer. The cPCR was performed using 1 cycle of 95 °C for 3 min; 35 cycles of 95 °C for 30 s, 62 °C for 30 s (for OI1–OI2c) or 58 °C for 30 s (for LSS–Las606) and 72 °C for 1 min (for OI1–OI2c) or for 40 s (LSS–Las606); followed by 1 cycle of 72 °C for 5 min. PCR products (5 µl) were analyzed on 1% agarose gel, and the remaining 45 µl was used for amplicon sequencing.
Tissue samples used for HLB diagnostic real-time PCR using TXCChlb
The efficacy of TXCChlb for HLB diagnosis was tested on root samples from 76 mature (> 10 years old) citrus trees (15 ‘Navel’ and 26 ‘Marrs’ sweet orange and 35 ‘Rio Red’ grapefruit trees grown in commercial orchards in Texas) that had previously tested positive for CLas by real-time PCR using leaf DNA extracts. All trees were grafted onto sour orange rootstocks. Leaf and fibrous root samples were collected from each tree for DNA preparation. Root DNA extracts were prepared following the procedure described in Supplementary Document 1. Leaf DNA was prepared from ca. 200 mg of pooled midrib sections excised from 6 to 8 leaves showing HLB-like symptoms with BioSprint kit (Qiagen, Hilden, Germany) following the manufacturer’s instruction.
In addition, leaf and fibrous root samples were collected from 21 young (4–5 years old) ‘Rio Red’ grapefruit trees from a commercial orchard in Donna, Texas in order to examine the usefulness of TXCChlb for HLB detection at pre-symptomatic stage. Before sampling for this study, these trees had not been tested for CLas infection. Among these trees, only two trees (Tree ID: a1 and a2) had phenotypes that could be confused for HLB symptoms, whether these phenotypes were due to nutrient deficiency or CLas infection was unknown at the time of sampling. For comparison, we sampled two neighboring mature (> 10 years old) ‘Rio Red’ grapefruit trees that had previously tested positive for CLas in real-time PCR using HLBaspr and leaf DNA.
Results
Multiple sequence analysis of 16S rDNA of various Candidatus Liberibacter species
Multiple sequence alignment revealed a few regions that are conserved among CLas strains, but not with other “Ca. Liberibacter” species (Fig. 1). These regions were analyzed to design real-time PCR primers and probe using Primer3 (Koressaar and Remm 2007) and by visual inspection, which led to the development of new real-time PCR primers based on CLas 16S rDNA (Fig. 1). To examine any potential cross-reactivity of the newly designed primer set, TXCChlb, with 16S rDNAs of other soil bacteria, we did a BLASTn search of the prokaryotic ribosomal RNA database after excluding the CLas 16S rDNA sequence (NCBI TaxID 34,102) and found that TXCChlb-f has ≤ 66% sequence coverage with 100% identity to the sequences on the prokaryotic 16S rDNA database, while TXCChlb-r has a maximum of 90% sequence coverage with 100% identity to the prokaryotic 16S rDNA database (data not shown). The predicted amplification efficiency of TXCChlb was ~ 97% (Fig. 2).
Specificity of TXCChlb
For examining the specificity of TXCChlb, DNA fractions that contain 16S rDNA of three “Ca. Liberibacter” species, CLas, CLam and CLaf, were obtained from the USDA APHIS Lab at Beltsville, MD and used for real-time PCR with TXCChlb following the PCR parameters described in Materials and Methods. The result (Fig. 3) showed that TXCChlb detected all three HLB causing “Ca. Liberibacter” species. The efficiency of TXCChlb for the detection of each of all three HLB causing bacteria, CLas, CLam and CLaf was tested using real-time PCR with a series of diluted template DNAs which were prepared from the DNA templates used for the reaction in Fig. 3. For comparison, real-time PCR primer sets targeting 16S rDNA of CLas (HLBaspr), CLam (HLBampr) and CLaf (HLBafpr) (Li et al. 2006) were included because their nonspecific amplification renders them unsuitable for HLB detection in root tissue (data not shown). The test results (Table 1) showed that the efficiency of TXCChlb for CLas detection is comparable to that of HLBaspr and that TXCChlb can detect CLam although it is slightly less efficient than HLBampr due to the nucleotide mismatch between TXCChlb and its target region in CLam 16S rDNA (Fig. 1). On the other hand, TXCChlb is not suitable for CLaf detection because it can only detect CLaf when the titer is high (Table 1). In the United States, HLB is caused by CLas; thus, all HLB-positive or -symptomatic samples tested in the current study are HLB-positive samples caused by CLas. In addition, because Table 1 indicated that HLB detection sensitivity of TXCChlb is comparable to that of HLBaspr, we adopted the same USDA APHIS guideline suggested for HLBaspr to consider samples with threshold cycle (Ct) less than 37 as HLB positive.
TXCChlb specificity test by real-time PCR using DNA fractions that contained 16S rDNA fragment of Candidatus Liberibacter asiaticus (CLas), Ca. L. americanus (CLam) and Ca. L. africanus (CLaf). a CLam (Ct = 21.13), b CLas (Ct = 22.63), c CLaf (Ct = 25.73), d negative control. Threshold is indicated in the figure
Efficacy of TXCChlb for HLB diagnosis using citrus fibrous root tissue
The efficacy of TXCChlb for HLB diagnosis was examined on the 76 citrus trees (see Materials and Methods) that had tested positive by real-time PCR using leaf DNA, together with HLBaspr (Li et al. 2006) and CQULA (Wang et al. 2006) for comparison. The HLB detection rate by real-time PCR with HLBaspr was ~ 88% for sweet orange and 100% for grapefruit leaves (Table 2). In comparison, the real-time PCR with TXCChlb using leaf DNA extracts revealed ~ 83 and 100% detection rates in sweet orange and grapefruit, respectively, while root samples tested with TXCChlb showed 100% positive detection in both sweet orange and grapefruit (Table 2). On the other hand, the CQULA system applied to leaf DNA extracts resulted in ~ 76 and ~ 97% detection in sweet orange and grapefruit, respectively, while root samples tested with CQULA showed ~ 98% detection in sweet orange and ~ 94% in grapefruit (Table 2). None of these three primer sets gave 100% detection of CLas using leaf DNA from the infected sweet orange trees (Table 2), perhaps due to erroneous leaf tissue sampling as a result of uneven distribution of aerial HLB symptoms and/or due to the similarity of HLB symptoms with various nutrient deficiencies. This data from HLB-positive mature trees suggested that (1) the detection rate for Liberibacter in leaves using TXCChlb is comparable to that with HLBaspr, (2) both HLBaspr and TXCChlb are more sensitive than CQULA, and (3) detection from root tissue using TXCChlb is more consistent than from leaves tested with HLBaspr.
Evaluation of HLB detection at pre-symptomatic stage using fibrous root tissue
The potential of TXCChlb for asymptomatic HLB diagnosis by real-time PCR was evaluated using tissue samples collected from 21 young (4–5 years old) grapefruit trees and two HLB-positive mature grapefruit trees (see Materials and Methods). Of 21 young trees, two trees (a1 and a2) were positive for CLas in leaf samples with all three primer-probe sets (HLBaspr, CQULA and TXCChlb), while four trees (a1, a2, a7 and a9) tested positive for root samples using CQULA and TXCChlb (Table 3). The two neighboring mature trees were positive for CLas with all primer-probe sets using leaf tissue (Table 3). Root tissue samples from these mature trees produced positive results for CLas using TXCChlb in both trees, while only one root sample tested positive by CQULA (Table 3), suggesting that TXCChlb is likely more sensitive than CQULA.
The real-time PCR data obtained with TXCChlb for those six CLas-positive root samples (Table 3) were then confirmed using cPCR and cPCR primer sets OI1/OI2c (Jagoueix et al. 1996) and LSS/Las606 (Fujikawa and Iwanami 2012), which target CLas 16S rDNA. cPCR data showed that primer set OI1/OI2c produces non-target amplicons along with target amplicon and failed to confirm the real-time PCR data by agarose gel electrophoresis (Fig. 4a). Thus, OI1/OI2c is not suitable for root cPCR, and these results agree with our previous reports (Kunta et al. 2014b; Louzada et al. 2016). On the other hand, primer set LSS/Las606 produced a single amplicon of ~ 500 bp in all six CLas positive root samples (Fig. 4b). The sequencing results showed that the nucleotide sequence of LSS-Las606 amplicons (GenBank ID: KY926796, KY926797) obtained from root samples completely matches the 16S rDNAs of CLas strains (e.g. CP010804, AP014595, CP004005 and CP001677). These data confirmed that those root samples that tested positive for CLas with TXCChlb are true positives and that HLB can be detected at a pre-symptomatic stage by using fibrous root tissue for the HLB test. In addition, the data in Table 3 showed that any potential cross-reactivity of TXCChlb to 16S rDNAs of other soil bacteria is negligible and that the root DNA extract prepared by the protocol described in Supplementary Document 1 is suitable for HLB detection by real-time PCR.
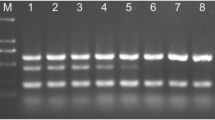
Agarose gel electrophoresis of conventional PCR amplicons obtained from HLB-positive root DNA. a OI1/OI2c amplicon. b LSS/Las606 amplicon. Lane M, DNA size markers. The tree ID is indicated at the top. The arrow and asterisk (*) in a indicate the OI1-OI2c amplicon and nonspecific amplicon, respectively
Robustness of root HLB test compared to leaf HLB test
To confirm the robustness of TXCChlb in the face of geographic, edaphic, and rootstock variation, TXCChlb was evaluated with leaf and root tissue samples collected in April 2016 from 99 sweet orange trees (4–5 years old) that were grown in Florida. The HLB diagnostic real-time PCR was conducted with HLBaspr for leaf and TXCChlb for root DNA extracts. Of 99 sweet orange trees, 17 trees tested positive for CLas with root samples, while only four trees were positive with leaf samples (Fig. 5a). Among these CLas-positive trees, two trees tested positive with both leaf and root tissue (Fig. 5a). cPCR conducted with LSS/Las606 (Fujikawa and Iwanami 2012) followed by agarose gel electrophoresis confirmed the presence of CLas 16S rDNA in 15 of 17 CLas positive root samples (Fig. 5b). The sequencing results showed that the nucleotide sequence of 501 bp LSS-Las606 amplicons (GenBank ID: KY926798) obtained from these 15 CLas-positive root samples was a 100% match to the CLas 16S rDNA. The Ct values of the two samples for which cPCR failed were 36.27 and 36.21, respectively, suggesting that Ct value of ~ 36 obtained with TXCChlb may be the cPCR detection limit for CLas 16S rDNA (Fig. 5b).
Comparison of Candidatus Liberibacter asiaticus (CLas) detection results obtained with Florida leaf and root tissue samples using HLBaspr and TXCChlb (a) and agarose gel electrophoresis of conventional PCR amplicons of 17 CLas positives obtained with TXCChlb (b). Leaf and root samples were collected from each of 99 sweet orange trees grown in Florida in April 2016. The primer-probe set and the tissue sample are indicated in the figure together with the number of CLas positives and the total number of samples. Asterisk indicates number of CLas positive/Total number of samples. The x in the agarose gel indicates samples (Ct = ~ 36) for which the conventional PCR failed
Discussion
HLB, one of the oldest known citrus diseases, has caused unprecedented economic losses worldwide (Bové 2006). Since the first report of HLB in Florida in 2005 (Halbert 2005; Bové 2006; Gottwald 2010), the Florida citrus industry has suffered almost 67.4% yield decrease in orange production between 2007/2008 and 2015/2016 seasons (Monzo and Stansly 2017), and in 2014, the net capital loss (producer plus consumer losses) in Florida due to HLB was estimated at ~$1 billion per year (Farnsworth et al. 2014). Despite the destructive nature of HLB in citriculture, neither an effective control strategy nor resistant varieties have been developed yet (Gottwald 2010). At present, citrus industry heavily depends on year-round chemical sprays to control the insect vector (psyllid) population and additional nutrient application to maximize the yield when HLB is widespread, thus greatly increasing production costs (Farnsworth et al. 2014). However, this strategy has only limited effect on controlling HLB spread because there has been no early HLB detection method developed yet, which can be useful for the removal of HLB positive trees at pre-symptomatic stage.
Currently, routine HLB diagnosis is conducted by visual inspection of a tree canopy for typical HLB symptoms; real-time PCR using DNA extracted from symptomatic leaves is merely a confirmation of HLB in a symptomatic tree. Since HLB symptom development is a slow process taking a few months to 1 or more years, it is highly likely that any infected tree without visible HLB symptoms will not be selected for testing and thus will serve as an inoculum source for HLB (Lee et al. 2015). Our group previously reported that HLB-causing bacteria are relatively even distributed in the citrus root system compared to the aerial part of a tree infected with CLas (Kunta et al. 2014a; Louzada et al. 2016), suggesting that the root tissue can be an alternative source material for routine HLB diagnosis. The current study was initiated to examine the root tissue as an alternative source material for HLB diagnosis. However, due to the non-specific amplification of HLBaspr, a widely used real-time PCR primer-probe set for HLB diagnosis, with root DNA fraction (Kunta et al. 2014b; Louzada et al. 2016), the current study first focused on the development of a new primer-probe set, TXCChlb, based on CLas 16 s rDNA and then optimized the root DNA preparation for PCR by incorporating a step to remove PCR inhibitors present in the root DNA fraction. The development and evaluation of primer-probe set TXCChlb using DNA from leaves and fibrous roots collected from young citrus trees in Texas and Florida confirmed that HLB can be detected using real-time PCR with TXCChlb from roots before symptoms appear aboveground. This early detection will help provide more effective HLB control, especially where HLB disease is not yet widespread. In addition, the use of TXCChlb with real-time PCR has potential for detecting CLam from fibrous root tissue. In regions where CLam coexists with CLas, the cPCR confirmation step is also required to confirm the identity of HLB-causing bacteria.
Change history
14 August 2018
The article “A new diagnostic real-time PCR method for huanglongbing detection in citrus root tissue”, written by Jong Won Park, Eliezer S. Louzada, W. Evan Braswell, Philip A. Stansly, John V. da Graça, Greg McCollum, John E. Rascoe, and Madhurababu Kunta, was originally published electronically on the publisher’s internet portal (currently SpringerLink) on 13 June 2018 without open access.
References
Bové JM (2006) Huanglongbing: a destructive, newly-emerging, century-old disease of citrus. J Plant Pathol 88:7–37
Capoor SP, Rao DG, Viswanath SM (1967) Diaphorina citri Kuway., a vector of the greening disease of citrus in India. Ind J Agric Sci 37:572–576
da Graça JV, Korsten L (2004) Citrus Huanglongbing: review, present status and future strategies. In: Naqvi SAMH (ed) Diseases of fruits and vegetables, vol 1. Springer, Dordrecht, Netherlands, pp 229–245
Farnsworth D, Grogan KA, van Bruggen AHC, Moss CB (2014) The potential economic cost and response to greening in Florida citrus. Choice 29:1–6
Fujikawa T, Iwanami T (2012) Sensitive and robust detection of citrus greening (huanglongbing) bacterium “Candidatus Liberibacter asiaticus” by DNA amplification with new 16S rDNA-specific primers. Mol Cell Probes 26:194–197
Gottwald TR (2010) Current epidemiological understanding of citrus Huanglongbing. Annu Rev Phytopathol 48:119–139
Gottwald TR, Parnell S, Taylor E, Poole K, Hodge J, Ford A, Therrien L, Mayo S, Irey M (2008) Within-tree distribution of Candidatus Liberibacter asiaticus. In: Gottwald TR, Graham JH (eds) Proceedings of the 1st International Research Conference on Huanglongbing. Florida Citrus Mutual, Orlando, FL, USA, pp 310–313
Halbert SE (2005) The discovery of huanglongbing in Florida. Proceedings of the international citrus canker and huanglongbing research workshop, Orlando, FL, USA, H-3 (Abstract)
Jagoueix S, Bové JM, Garnier M (1994) The phloem-limited bacterium of greening disease of citrus is a member of the α subdivision of the Proteobacteria. Int J Syst Bacteriol 44:379–386
Jagoueix S, Bové JM, Garnier M (1996) PCR detection of the two ‘Candidatus’ liberobacter species associated with greening disease of citrus. Mol Cell Probes 10:43–50
Johnson EG, Wu J, Bright DB, Graham JH (2014) Association of ‘Candidatus Liberibacter asiaticus’ root infection, but not phloem plugging with root loss on huanglongbing-affected trees prior to appearance of foliar symptoms. Plant Pathol 63:290–298
Koressaar T, Remm M (2007) Enhancements and modifications of primer design program Primer3. Bioinformatics 23:1289–1291
Kunta M, da Graça JV, Malik N, Louzada ES, Sétamou M (2014a) Quantitative distribution of Candidatus Liberibacter asiaticus in the aerial parts of the Huanglongbing-infected citrus trees in Texas. HortScience 49:65–68
Kunta M, Viloria Z, del Rio HS, Louzada ES (2014b) Diverse DNA extraction methods and PCR primers for detection of Huanglongbing-associated bacteria from roots of ‘Valencia’ sweet orange on sour orange rootstock. Scientia Horticult 178:23–30
Lee JA, Halbert SE, Dawson WO, Robertson CJ, Keesling JE, Singer BH (2015) Asymptomatic spread of huanglongbing and implications for disease control. Proc Natl Acad Sci USA 112:7605–7610
Li W, Hartung JS, Levy L (2006) Quantitative real-time PCR for detection and identification of Candidatus Liberibacter species associated with citrus huanglongbing. J Microbiol Methods 66:104–115
Louzada ES, Vazquez OE, Braswell WE, Yanev G, Devanaboina M, Kunta M (2016) Distribution of ‘Candidatus Liberibacter asiaticus’ above and below ground in Texas citrus. Phytopathology 106:702–709
McClean APD, Oberholzer PCJ (1965) Citrus psylla, a vector of the greening disease of sweet orange. S Afr J Agric Sci 8:297–298
Monzo C, Stansly PA (2017) Economic injury levels for Asian citrus psyllid control in process oranges from mature trees with high incidence of huanglongbing. PLoS One 12:e0175333
Morgan JK, Zhou L, Li W, Shatters RG, Keremane M, Duan YP (2012) Improved real-time PCR detection of ‘Candidatus Liberibacter asiaticus’ from citrus and psyllid hosts by targeting the intragenic tandem-repeats of its prophage genes. Mol Cell Probes 26:90–98
Planet P, Jagoueix S, Bové JM, Garnier M (1995) Detection and characterization of the African citrus greening liberobacter by amplification, cloning, and sequencing of the rplKAJL-rpoBC operon. Curr Microbiol 30:137–141
Shen W, Halbert SE, Dickstein E, Manjunath KL, Shimwela MM, van Bruggen AHC (2013) Occurrence and in-grove distribution of citrus huanglongbing in north central Florida. J Plant Pathol 95:361–371
Teixeira DC, Ayres AJ, Danet JL, Saillard ES, Bové JM (2005) First report of a huanglongbing-like disease of citrus in Sao Paulo State, Brazil, and association of a new Liberibacter species, “Candidatus Liberibacter americanus”, with the disease. Plant Dis 89:107
Wang N, Trivedi P (2013) Citrus huanglongbing: a newly relevant disease presents unprecedented challenges. Phytopathology 103:652–665
Wang Z, Yin Y, Hu H, Yuan Q, Peng G, Xia Y (2006) Development and application of molecular-based diagnosis for ‘Candidatus Liberibacter asiaticus’, the causal pathogen of citrus huanglongbing. Plant Pathol 55:630–638
Yarza P, Ludvig W, Euzéby J, Amann R, Schleifer KH, Glöckner FO, Rosselló-Móra R (2010) Update of the all-species living tree project based on 16S and 23S rRNA sequence analyses. Sys Appl Microbiol 33:291–299
Zheng Z, Xu M, Bao M, Wu F, Chen J, Deng X (2016) Unusual five copies and dual forms of nrdB in “Candidatus Liberibacter asiaticus”: Biological implications and PCR detection application. Sci Rep 6:39020
Acknowledgements
We greatly thank Jim Brockington (Citrus Center, Texas A&M Univ.-Kingsville, Weslaco, TX) and Barry C. Kostyk (University of Florida-IFAS, Southwest Florida Research and Education Center, Immokalee, FL) for collecting and processing tissue samples. We also thank Jennifer Trevino at Wonderful Citrus in Edinburg, Texas for her help with tissue sampling and Yessica Cerino at the Citrus Center, Texas A&M Univ.-Kingsville, for reviewing the manuscript. This work was supported by funds from USDA-APHIS PPQ, USDA MAC cooperative agreement #15-8130-0489-CA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
The original version of this article was revised due to a retrospective Open Access order.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10327_2018_793_MOESM1_ESM.docx
Supplementary Document 1. Protocol for citrus root DNA preparation from fibrous root tissue and PCR inhibitor removal (DOCX 44 KB)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
About this article
Cite this article
Park, JW., Louzada, E.S., Braswell, W.E. et al. A new diagnostic real-time PCR method for huanglongbing detection in citrus root tissue. J Gen Plant Pathol 84, 359–367 (2018). https://doi.org/10.1007/s10327-018-0793-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10327-018-0793-4