Abstract
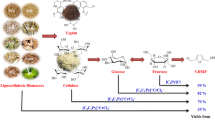
We report for the first time the direct conversion of raw grape berry biomass to hydroxymethylfurfural (HMF) using ionic liquid solvents with metal chloride catalysts. Exploiting raw plant biomass as a biorefinery feedstock is innovative for sustainable chemical industry. The use of the raw biomass to synthesize compounds can indeed lead to less energy consumption and less CO2 emissions. Using raw plant biomass skips pretreatment steps that are required to produce biomaterials such as carbohydrates or cellulosic biomass. Here, grape berry biomass was used as a raw chemical feedstock for the production of hydroxymethylfurfural, a key platform intermediate for syntheses of future renewable biofuels. We examined 3 ionic liquid solvents, 3 reaction temperatures, 5 chloride catalysts, and 5 concentrations of HCl. We found an increasing HMF yields depending on reaction conditions. 1-octyl-3-methylimidazolium chloride was most effective for HMF synthesis. Addition of HCl or metal chlorides alone showed little improvement. The highest HMF yield of about 100 mg HMF per mL of grape biomass extract was obtained using 0.3 M HCl, [OMIM]Cl, and CrCl2 at 100°C for 3 h. Our study provides a model system of sustainable production of valuable compounds from raw plant biomass.





Similar content being viewed by others
References
Antal MJ, Mok WSL, Richards GN (1990) Mechanism of formation of 5-(hydroxymethyl)-2-furaldehyde from d-fructose and sucrose. Carbohydr Res 199:91–109
Black M, Miller RJ (2006) Perspective platform chemicals from crops. Chem Technol Biotechnol 81:1725–1728
Cazor A, Deborde C, Moing A, Rolin D, This H (2006) Sucrose, glucose, and fructose extraction in aqueous carrot root extracts prepared at different temperatures by means of direct NMR measurements. J Agricul Food Chem 54:4681–4686
Chun J-A, Lee J-W, Yi Y-B, Hong S–S, Chung C-H (2010a) Catalytic production of hydroxymethylfurfural from sucrose using 1-octyl-3 methylimidazolium chloride ionic liquid. Kor J Chem Eng 27:920–925
Chun J-A, Lee J-W, Yi Y-B, Hong S–S, Chung C-H (2010b) Direct conversion of starch to hydroxymethylfurfural in the presence of an ionic liquid with metal chloride. Starch/Stärke 62:326–330
Lee J-W, Ha M-K, Yi Y-B, Chung C-H (2011) Chromium halides mediated production of hydroxymethylfurfural from starch-rich acorn biomass in an acidic ionic liquid. Carbohydr Res 346:177–182
Lewkowski J (2001) Synthesis, chemistry, and applications of 5-hydroxymethyl-furfural and its derivatives. ARKIVOC,1, (ARKAT-USA;ISSN1424-6376), pp 17. http://www.arkat-usa.org/home.aspx?VIEW-MANUSCRIPT&MSID=403
Moreau C, Finiels A, Vanoye L (2006) Dehydration of fructose and sucrose into 5-hydroxymethylfurfural in the presence of 1-H-3-methyl imidazolium chloride acting both as solovent and catalyst. J Mol Catal A Chem 253:165–169
Olivier-Bourbigou H, Magna L, Morvan D (2010) Ionic liquid and catalysis: recent progress from knowledge to applications. Appl Catal A Gen 373:1–56
Qi X, Watanabe M, Aida TM, Smith RL Jr (2009) Efficient catalytic conversion of fructose into 5-hydroxymethylfurfural in ionic liquids at room temperature. ChemSusChem 2:944–946
Ragauskas AJ, Williams CK, Davison BH, Britovsek G, Cainey J, Eckert CA, Federick WJ Jr, Hallet JP, Leak DJ, Liotta CL, Mielenz JR, Murphy R, Templer R, Tschaplinski T (2006) The path forward for biofuels and biomaterials. Science 311:484–489
Román-Leshkov Y, Barrett CJ, Liu ZY, Dumesic JA (2007) Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates. Nature 447:982–986
Su Y, Brown HM, Huang X, Zhou X-D, Amonette JE, Zhang ZC (2009) Single-step conversion of cellulose to 5-ydroxymethylfurfural (HMF), a versatile platform chemical. Appl Catal A Gen 361:117–122
Yan L, Yang N, Pang H, Liao B (2008) Production of levulinic acid from bagasse and paddy straw by liquefaction in the presence of hydrochloric acid. Clean-Soil Air Water 36:158–163
Yi Y-B, Lee J-W, Hong S–S, Choi Y-H, Chung C-H (2011) Acid-mediated production of hydroxymethylfurfural from raw plant biomass with high inulin in an ionic liquid. J Ind Eng Chem 17:6–9
Youngs TGA, Hardacre C, Holbrey JD (2007) Glucose solvation by the ionic liquid 1, 3 dimethylimidasolium chloride: A simulation study. J Phy Chem B 111:13765–13774
Zhao H, Holladay JE, Brown H, Zhang ZC (2007) Metal chlorides in ionic liquid solvents convert sugars to 5-hydroxymethylfurfural. Science 316:1597–1600
Acknowledgments
This study was supported by the Dong-A University Research Fund. The authors acknowledge the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yi, YB., Lee, JL., Choi, YH. et al. Direct production of hydroxymethylfurfural from raw grape berry biomass using ionic liquids and metal chlorides. Environ Chem Lett 10, 13–19 (2012). https://doi.org/10.1007/s10311-011-0322-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-011-0322-6