Abstract
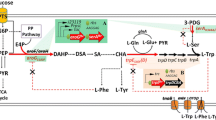
l-tryptophan (l-trp) is a precursor of various bioactive components and has great pharmaceutical interest. However, due to the requirement of several precursors and complex regulation of the pathways involved, the development of an efficient l-trp production strain is challenging. In this study, Escherichia coli (E. coli) strain KW001 was designed to overexpress the l-trp operator sequences (trpEDCBA) and 3-deoxy-D-arabinoheptulosonate-7-phosphate synthase (aroGfbr). To further improve the production of l-trp, pyruvate kinase (pykF) and the phosphotransferase system HPr (ptsH) were deleted after inactivation of repression (trpR) and attenuation (attenuator) to produce strain KW006. To overcome the relatively slow growth and to increase the transport rate of glucose, strain KW018 was generated by combinatorial regulation of glucokinase (galP) and galactose permease (glk) expression. To reduce the production of acetic acid, strain KW023 was created by repressive regulation of phosphate acetyltransferase (pta) expression. In conclusion, strain KW023 efficiently produced 39.7 g/L of l-trp with a conversion rate of 16.7% and a productivity of 1.6 g/L/h in a 5 L fed-batch fermentation system.






Similar content being viewed by others
References
Aiba S, Tsunekawa H, Imanaka T (1982) New approach to tryptophan production by Escherichia coli: genetic manipulation of composite plasmids in vitro. Appl Environ Microbiol 43:289–297
Bongaerts J, Kramer M, Muller U, Raeven L, Wubbolts M (2001) Metabolic engineering for microbial production of aromatic amino acids and derived compounds. Metab Eng 3:289–300. https://doi.org/10.1006/mben.2001.0196
Chan E-C, Tsai H-L, Chen S-L, Mou D-G (1993) Amplification of the tryptophan operon gene in Escherichia coli chromosome to increase l-tryptophan biosynthesis. Appl Microbiol Biotechnol 40:301–305. https://doi.org/10.1007/bf00170384
Chandran SS, Yi J, Draths KM, Von DR, Weber W, Frost JW (2003) Phosphoenolpyruvate availability and the biosynthesis of shikimic acid. Biotechnol Prog 19:808–814
Cheng L-K, Wang J, Xu Q-Y, Xie X-X, Zhang Y-J, Zhao C-G, Chen N (2012) Effect of feeding strategy on l-tryptophan production by recombinant Escherichia coli. Ann of Microbiology 62:1625–1634. https://doi.org/10.1007/s13213-012-0419-6
de la Cueva-Mendez G, Pimentel B (2007) Gene and cell survival: lessons from prokaryotic plasmid R1. EMBO Rep 8:458–464. https://doi.org/10.1038/sj.embor.7400957
de Smit MH, van Duin J (1994) Control of translation by mRNA secondary structure in Escherichia coli. A quantitative analysis of literature data. J Mol Biol 244:144–150. https://doi.org/10.1006/jmbi.1994.1714
Dodge TC, Gerstner JM (2002) Optimization of the glucose feed rate profile for the production of tryptophan from recombinant E coli. J Chem Technol Biotechnol 77:1238–1245
Flores N, Xiao J, Berry A, Bolivar F, Valle F (1996) Pathway engineering for the production of aromatic compounds in Escherichia coli. Nat Biotechnol 14:620–623. https://doi.org/10.1038/nbt0596-620
Ger YM, Chen SL, Chiang HJ, Shiuan D (1994) A single Ser-180 mutation desensitizes feedback inhibition of the phenylalanine-sensitive 3-deoxy-D-arabino-heptulosonate 7-phosphate (DAHP) synthetase in Escherichia coli. J Biochem 116:986–990
Gu P, Yang F, Kang J, Wang Q, Qi Q (2012) One-step of tryptophan attenuator inactivation and promoter swapping to improve the production of l-tryptophan in Escherichia coli. Microb Cell Fact 11:30. https://doi.org/10.1186/1475-2859-11-30
Gu P, Yang F, Li F, Liang Q, Qi Q (2013) Knocking out analysis of tryptophan permeases in Escherichia coli for improving l-tryptophan production. Appl Microbiol Biotechnol 97:6677–6683. https://doi.org/10.1007/s00253-013-4988-5
Gunsalus RP, Yanofsky C (1980) Nucleotide sequence and expression of Escherichia coli trpR, the structural gene for the trp aporepressor. Proc Natl Acad Sci USA 77:7117–7121
Han K, Lim HC, Hong J (1992) Acetic acid formation in Escherichia coli fermentation. Biotechnol Bioeng 39:663–671. https://doi.org/10.1002/bit.260390611
Hernández-Montalvo V, Martínez A, Hernández-Chavez G, Bolivar F, Valle F, Gosset G (2003) Expression of galP and glk in a Escherichia coli PTS mutant restores glucose transport and increases glycolytic flux to fermentation products. Biotechnol Bioeng 83:687–694
Ikeda M (2006) Towards bacterial strains overproducing l-tryptophan and other aromatics by metabolic engineering. Appl Microbiol Biotechnol 69:615–626. https://doi.org/10.1007/s00253-005-0252-y
Klein AH, Shulla A, Reimann SA, Keating DH, Wolfe AJ (2007) The intracellular concentration of acetyl phosphate in Escherichia coli is sufficient for direct phosphorylation of two-component response regulators. J Bacteriol 189:5574–5581. https://doi.org/10.1128/jb.00564-07
Klig LS, Carey J, Yanofsky C (1988) trp repressor interactions with the trp aroH and trpR operators. Comparison of repressor binding in vitro and repression in vivo. J Mol Biol 202:769–777
Laalami S, Putzer H (2011) mRNA degradation and maturation in prokaryotes: the global players. Biomol Concepts 2:491–506. https://doi.org/10.1515/bmc.2011.042
Leuchtenberger W, Huthmacher K, Drauz K (2005) Biotechnological production of amino acids and derivatives: current status and prospects. Appl Microbiol Biotechnol 69:1–8. https://doi.org/10.1007/s00253-005-0155-y
Liu L, Duan X, Wu J (2016) l-Tryptophan Production in Escherichia coli Improved by weakening the Pta-AckA Pathway. PLoS One 11:e0158200. https://doi.org/10.1371/journal.pone.0158200
Meza E, Becker J, Bolivar F, Gosset G, Wittmann C (2012) Consequences of phosphoenolpyruvate:sugar phosphotranferase system and pyruvate kinase isozymes inactivation in central carbon metabolism flux distribution in Escherichia coli. Microb Cell Fact 11:127. https://doi.org/10.1186/1475-2859-11-127
Muñoz ME, Ponce E (2003) Pyruvate kinase: current status of regulatory and functional properties. Comp Biochem Physiol B Biochem Mol Biol 135:197
Patnaik R, Liao JC (1994) Engineering of Escherichia coli central metabolism for aromatic metabolite production with near theoretical yield. Appl Environ Microbiol 60:3903–3908
Postma PW, Lengeler JW, Jacobson GR (1993) Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol Rev 57:543–594
Rodriguez A, Martinez JA, Baez-Viveros JL, Flores N, Hernandez-Chavez G, Ramirez OT, Gosset G, Bolivar F (2013) Constitutive expression of selected genes from the pentose phosphate and aromatic pathways increases the shikimic acid yield in high-glucose batch cultures of an Escherichia coli strain lacking PTS and pykF. Microb Cell Fact 12:86. https://doi.org/10.1186/1475-2859-12-86
Sabido A, Sigala JC, Hernandez-Chavez G, Flores N, Gosset G, Bolivar F (2014) Physiological and transcriptional characterization of Escherichia coli strains lacking interconversion of phosphoenolpyruvate and pyruvate when glucose and acetate are coutilized. Biotechnol Bioeng 111:1150–1160. https://doi.org/10.1002/bit.25177
Sadeghiyan-Rizi T, Fooladi J, Sadrai S (2016) Preliminary study on cost-effective l-Tryptophan production from indole and l-Serine by E. coli cells. Avicenna J Med Biotechnol 8:188–192
Sarsero JP, Wookey PJ, Pittard AJ (1991) Regulation of expression of the Escherichia coli K-12 mtr gene by TyrR protein and Trp repressor. J Bacteriol 173:4133–4143
Schuster S, Dandekar T, Fell DA (1999) Detection of elementary flux modes in biochemical networks: a promising tool for pathway analysis and metabolic engineering. Trends Biotechnol 17:53–60
Shen T, Liu Q, Xie X, Xu Q, Chen N (2012) Improved production of tryptophan in genetically engineered Escherichia coli with TktA and PpsA overexpression. J Biomed Biotechnol 2012:605219. https://doi.org/10.1155/2012/605219
Shi A, Zhu X, Lu J, Zhang X, Ma Y (2013) Activating transhydrogenase and NAD kinase in combination for improving isobutanol production. Metab Eng 16:1–10. https://doi.org/10.1016/j.ymben.2012.11.008
Tang J, Zhu X, Lu J, Liu P, Xu H, Tan Z, Zhang X (2013) Recruiting alternative glucose utilization pathways for improving succinate production. Appl Microbiol Biotechnol 97:2513–2520. https://doi.org/10.1007/s00253-012-4344-1
Wang J, Cheng LK, Wang J, Liu Q, Shen T, Chen N (2013) Genetic engineering of Escherichia coli to enhance production of l-tryptophan. Appl Microbiol Biotechnol 97:7587–7596. https://doi.org/10.1007/s00253-013-5026-3
Wang Q, Wu C, Chen T, Chen X, Zhao X (2006) Expression of galactose permease and pyruvate carboxylase in Escherichia coli ptsG mutant increases the growth rate and succinate yield under anaerobic conditions. Biotech Lett 28:89–93. https://doi.org/10.1007/s10529-005-4952-2
Yoo SM, Na D, Lee SY (2013) Design and use of synthetic regulatory small RNAs to control gene expression in Escherichia coli. Nat Protoc 8:1694–1707. https://doi.org/10.1038/nprot.2013.105
Zhang X, Jantama K, Moore JC, Jarboe LR, Shanmugam KT, Ingram LO (2009) Metabolic evolution of energy-conserving pathways for succinate production in Escherichia coli. Proc Natl Acad Sci USA 106:20180–20185. https://doi.org/10.1073/pnas.0905396106
Zhao D, Yuan S, Xiong B, Sun H, Ye L, Li J, Zhang X, Bi C (2016) Development of a fast and easy method for Escherichia coli genome editing with CRISPR/Cas9. Microb Cell Fact 15:205. https://doi.org/10.1186/s12934-016-0605-5
Zhao Y, Wang CS, Li FF, Liu ZN, Zhao GR (2016) Targeted optimization of central carbon metabolism for engineering succinate production in Escherichia coli. BMC Biotechnol 16:52. https://doi.org/10.1186/s12896-016-0284-7
Zhao ZJ, Zou C, Zhu YX, Dai J, Chen S, Wu D, Wu J, Chen J (2011) Development of l-tryptophan production strains by defined genetic modification in Escherichia coli. J Ind Microbiol Biotechnol 38:1921–1929. https://doi.org/10.1007/s10295-011-0978-8
Acknowledgements
This study was supported by the Tianjin Science Fund for Distinguished Young Scholars (17JCJQJC45300), the Nature Science Foundation of Tianjin City (CN) (16JCYBJC23500), Tianjin science and technology Project (15PTCYSY00020), the Key Projects in the Tianjin Science & Technology Pillar Program (14ZCZDSY00058), and Key Laboratory of Systems Microbial Biotechnology, Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests. All authors have agreed to the submission this manuscript to the “Journal of Industrial Microbiology and Biotechnology”.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, Y., Liu, Y., Ding, D. et al. Rational design and analysis of an Escherichia coli strain for high-efficiency tryptophan production. J Ind Microbiol Biotechnol 45, 357–367 (2018). https://doi.org/10.1007/s10295-018-2020-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-018-2020-x