Abstract
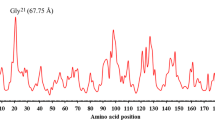
To improve the thermostability of a mesophilic GH family 10 xylanase, AuXyn10A, from Aspergillus usamii E001, its modification was performed by in silico design. Based on the comparison of B-factor values, a mutant xylanase ATXyn10 was predicted by substituting a segment YP from Tyr25 to Pro34 of AuXyn10A with the corresponding one from Asn24 to Ala32 of TaXyn10, a thermophilic GH family 10 xylanase from Thermoascus aurantiacus. Analysis of a TaXyn10 crystal structure indicated that there is a close interaction between segments YP and FP. For that reason, another mutant xylanase ATXyn10M was designed by mutating Ser286 and His288 of ATXyn10 into the corresponding Gly285 and Phe287 in the FP of TaXyn10. Then, two ATXyn10- and ATXyn10M-encoding genes, ATxyn10 and ATxyn10 M, were expressed in Pichia pas toris GS115. The temperature optimum of recombinant (re) ATXyn10M was 60 °C, 10 °C higher than that of reAuXyn10A. Its thermal inactivation half-life (t 1/2) at 55 °C was 10.4-fold longer than that of reAuXyn10A. As compared with reAuXyn10A, reATXyn10M displayed a slight decrease in K m value and a significant increase in V max value from 6,267 to 8,870 U/mg.








Similar content being viewed by others
References
Ahmed S, Riaz S, Jamil A (2009) Molecular cloning of fungal xylanases: an overview. Appl Microbiol Biotechnol 84:19–35
Badieyan S, Bevan DR, Zhang CM (2012) Study and design of stability in GH5 cellulases. Biotechnol Bioeng 109:31–44
Biely P, Vršanská M, Tenkanen M, Kluepfel D (1997) Endo-β-1,4-xylanase families: differences in catalytic properties. J Biotechnol 57:151–166
Cai HY, Shi PJ, Bai YG, Huang HQ, Yuan TZ, Yang PL, Luo HY, Meng K, Yao B (2011) A novel thermoacidophilic family 10 xylanase from Penicillium pinophilum C1. Process Biochem 46:2341–2346
Collins T, Gerday C, Feller G (2005) Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol Rev 29:2–23
Dumon C, Varvak A, Wall MA, Flint JE, Lewis RJ, Lakey JH, Morland C, Luginbuhl P, Healey S, Todaro T, DeSantis G, Sun M, Parra-Gessert L, Tan X, Weiner DP, Gilbert HJ (2008) Engineering hyperthermostability into a GH11 xylanase is mediated by subtle changes to protein structure. J Biol Chem 283:22557–22564
Ertl P, Rohde B, Selzer P (2000) Fast calculation of molecular polar surface area as a sum of fragment based contributions and its application to the prediction of drug transport properties. J Med Chem 43:3714–3717
Eswar N, Eramian D, Webb B, Shen MY, Sali A (2008) Protein structure modeling with MODELLER. Methods Mol Biol 426:145–159
Gao SJ, Wang JQ, Wu MC, Zhang HM, Yin X, Li JF (2013) Engineering hyperthermostability into a mesophilic family 11 xylanase from Aspergillus oryzae by in silico design of N-terminus substitution. Biotechnol Bioeng 110:1028–1038
Hakulinen N, Turunen O, Janis J, Leisola M, Rouvinen J (2003) Three-dimensional structures of thermophilic β-1,4-xylanases from Chaetomium thermophilum and Nonomuraea flexuosa: comparison of twelve xylanases in relation to their thermal stability. Eur J Biochem 270:1399–1412
Jaenicke R, Böhm G (1998) The stability of proteins in extreme environments. Curr Opin Struct Biol 8:738–748
Kabsch W, Sander C (1983) Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 22:2577–2637
Knapp S, Kardinahl S, Hellgren N, Tibbelin G, Schäfer G, Ladenstein R (1999) Refined crystal structure of a superoxide dismutase from hyperthermophilic archaeon Sulfolobus acidocaldarius at 2.2 Å resolution. J Mol Biol 285:689–702
Li JF, Tang CD, Shi HL, Wu MC (2011) Cloning and optimized expression of a neutral endoglucanase gene (ncel5A) from Volvariella volvacea WX32 in Pichia pastoris. J Biosci Bioeng 111:537–540
Lo Leggio L, Kalogiannis S, Bhat MK, Pickersgill RW (1999) High resolution structure and sequence of T. aurantiacus xylanase I: implications for the evolution of thermostability in family 10 xylanases and enzymes with (beta)alpha-barrel architecture. Proteins 36:295–306
Luo HY, Yang J, Li J, Shi PJ, Huang HQ, Bai YG, Fan YL, Yao B (2010) Molecular cloning and characterization of the novel acidic xylanase XYLD from Bispora sp. MEY-1 that is homologous to family 30 glycosyl hydrolases. Appl Microbiol Biotechnol 86:1829–1839
Miyazaki K, Takenouchi M (2002) Creating random mutagenesis libraries using megaprimer PCR of whole plasmid. Biotechniques 33:1033–1034
Pastor FIJ, Gallardo O, Sanz-Aparicio J, Diaz P (2007) Xylanases: molecular properties and applications. In: Polaina J, MacCabe AP (eds) Industrial enzymes. Springer, Germany, pp 65–82
Purmonen M, Valjakka J, Takkinen K, Laitinen T, Rouvinen J (2007) Molecular dynamics studies on the thermostability of family 11 xylanases. Protein Eng Des Sel 20:551–559
Radestock S, Gohlke H (2008) Exploiting the link between protein rigidity and thermostability for data-driven protein engineering. Eng Life Sci 8:507–522
Reetz MT, Carballeira JD (2007) Iterative saturation mutagenesis (ISM) for rapid directed evolution of functional enzymes. Nat Protoc 2:891–903
Reetz MT, Carballeira JD, Vogel A (2006) Iterative saturation mutagenesis on the basis of B factors as a strategy for increasing protein thermostability. Angew Chem Int Ed 45:7745–7751
Sunna A, Bergquist PL (2003) A gene encoding a novel extremely thermostable 1,4-β-xylanase isolated directly from an environmental DNA sample. Extremophiles 7:63–70
Vardakou M, Flint J, Christakopoulos P, Lewis RJ, Gilbert HJ, Murray JW (2005) A family 10 Thermoascus aurantiacus xylanase utilizes arabinose decorations of xylan as significant substrate specificity determinants. J Mol Biol 352:1060–1067
Vieille C, Zeikus GJ (2001) Hyperthermophilic enzymes: sources, uses, and molecular mechanisms for thermostability. Microbiol Mol Biol Rev 65:1–43
Wang JQ, Yin X, Wu MC, Zhang HM, Gao SJ, Wei JT, Tang CD, Li JF (2013) Expression of a family 10 xylanase gene from Aspergillus usamii E001 in Pichia pastoris and characterization of the recombinant enzyme. J Ind Microbiol Biotechnol 40:75–83
Wang JQ, Zhang HM, Wu MC, Tang CD (2011) Cloning and sequence analysis of a novel xylanase gene, Auxyn10A, from Aspergillus usamii. Biotechnol Lett 33:1029–1038
Yoon HS, Han NS, Kim CH (2004) Expression of Thermotoga maritima endo-β-1,4-xylanase gene in E. coli and characterization of the recombinant enzyme. Agric Chem Biotechnol 47:157–160
Zhang HM, Li JF, Wang JQ, Yang YJ, Wu MC (2014) Determinants for the improved thermostability of a mesophilic family 11 xylanase predicted by computational methods. Biotechnol Biofuels 7:3
Acknowledgments
This work was supported by the National Nature Science Foundation of China (No. 31101229), Key Laboratory of Carbohydrate Chemistry and Biotechnology, Ministry of Education, Jiangnan University (No. KLCCB-KF201208), Doctoral Research Funds of Jiangnan University (No. JUDCF11032) and Postgraduate Innovation Training Project of Jiangsu (No. CXZZ12_0758). The authors are grateful to Prof. Xianzhang Wu (School of Biotechnology, Jiangnan University) for providing technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
J. Wang and Z. Tan, the two first authors, contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, J., Tan, Z., Wu, M. et al. Improving the thermostability of a mesophilic family 10 xylanase, AuXyn10A, from Aspergillus usamii by in silico design. J Ind Microbiol Biotechnol 41, 1217–1225 (2014). https://doi.org/10.1007/s10295-014-1463-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-014-1463-y