Abstract
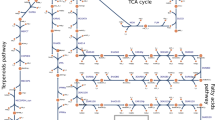
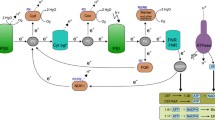
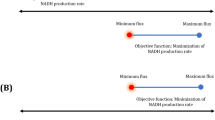
Synechocystis sp. PCC 6803 has been considered as a promising biocatalyst for electricity generation in recent microbial fuel cell research. However, the innate maximum current production potential and underlying metabolic pathways supporting the high current output are still unknown. This is mainly due to the fact that the high-current production cell phenotype results from the interaction among hundreds of reactions in the metabolism and it is impossible for reductionist methods to characterize the pathway selection in such a metabolic state. In this study, we employed computational metabolic techniques, flux balance analysis, and flux variability analysis, to exploit the maximum current outputs of Synechocystis sp. PCC 6803, in five electron transfer cases, namely, ferredoxin- and plastoquinol-dependent electron transfers under photoautotrophic cultivation, and NADH-dependent mediated electron transfer under photoautotrophic, heterotrophic, and mixotrophic conditions. In these five modes, the maximum current outputs were computed as 0.198, 0.7918, 0.198, 0.4652, and 0.4424 A gDW−1, respectively. Comparison of the five operational modes suggests that plastoquinol-/c-type cytochrome-targeted electricity generation had an advantage of liberating the highest current output achievable for Synechocystis sp. PCC 6803. On the other hand, the analysis indicates that the currency metabolite, NADH-, dependent electricity generation can rely on a number of reactions from different pathways, and is thus more robust against environmental perturbations.




Similar content being viewed by others
References
Atsumi S, Higashide W, Liao JC (2009) Direct photosynthetic recycling of carbon dioxide to isobutyraldehyde. Nat Biotechnol 27(12):1177–1180. doi:10.1038/nbt.1586
Babanova S, Hubenova Y, Mitov M (2011) Influence of artificial mediators on yeast-based fuel cell performance. J Biosci Bioeng 112(4):379–387. doi:10.1016/j.jbiosc.2011.06.008
Bhaya D (2004) Light matters: phototaxis and signal transduction in unicellular cyanobacteria. Mol Microbiol 53(3):745–754. doi:10.1111/j.1365-2958.2004.04160.x
Boesen T, Nielsen LP (2013) Molecular dissection of bacterial nanowires. MBio 4(3). doi:10.1128/mBio.00270-13
Chang RL, Ghamsari L, Manichaikul A, Hom EFY, Balaji S, Fu W, Shen Y, Hao T, Palsson BO, Salehi-Ashtiani K, Papin JA (2011) Metabolic network reconstruction of Chlamydomonas offers insight into light-driven algal metabolism. Mol Syst Biol 7. URL http://www.nature.com/msb/journal/v7/n1/suppinfo/msb201152_S1.html
Cheetham NWH (2011) Introducing biological energetics: how energy and information control the living. World Oxford University Press, New York
Cheng S, Liu H, Logan BE (2006) Increased power generation in a continuous flow MFC with advective flow through the porous anode and reduced electrode spacing. Environ Sci Technol 40(7):2426–2432. doi:10.1021/es051652w
Du Z, Li H, Gu T (2007) A state of the art review on microbial fuel cells: a promising technology for wastewater treatment and bioenergy. Biotechnol Adv 25(5):464–482. doi:10.1016/j.biotechadv.2007.05.004
Dutta D, De D, Chaudhuri S, Bhattacharya SK (2005) Hydrogen production by Cyanobacteria. Microb Cell Fact 4:36. doi:10.1186/1475-2859-4-36
Ekins S, Honeycutt JD, Metz JT (2010) Evolving molecules using multi-objective optimization: applying to ADME/Tox. Drug Discov Today 15(11–12):451–460. doi:10.1016/j.drudis.2010.04.003
Feng X, Xu Y, Chen Y, Tang YJ (2012) Integrating flux balance analysis into kinetic models to decipher the dynamic metabolism of Shewanella oneidensis MR-1. PLoS Comput Biol 8(2):e1002376. doi:10.1371/journal.pcbi.1002376
Gorby YA, Yanina S, McLean JS, Rosso KM, Moyles D, Dohnalkova A, Beveridge TJ, Chang IS, Kim BH, Kim KS, Culley DE, Reed SB, Romine MF, Saffarini DA, Hill EA, Shi L, Elias DA, Kennedy DW, Pinchuk G, Watanabe K, Ishii S, Logan B, Nealson KH, Fredrickson JK (2006) Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc Natl Acad Sci USA 103(30):11358–11363. doi:10.1073/pnas.0604517103
Gorby YA, Yanina S, McLean JS, Rosso KM, Moyles D, Dohnalkova A, Beveridge TJ, Chang IS, Kim BH, Kim KS, Culley DE, Reed SB, Romine MF, Saffarini DA, Hill EA, Shi L, Elias DA, Kennedy DW, Pinchuk G, Watanabe K, Ishii SÄ, Logan B, Nealson KH, Fredrickson JK (2006) Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc Natl Acad Sci 103(30):11358–11363. doi:10.1073/pnas.0604517103
Johnson CH, Stewart PL, Egli M (2011) The cyanobacterial circadian system: from biophysics to bioevolution. Annu Rev Biophys 40:143–167. doi:10.1146/annurev-biophys-042910-155317
Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, Kimura T, Hosouchi T, Matsuno A, Muraki A, Nakazaki N, Naruo K, Okumura S, Shimpo S, Takeuchi C, Wada T, Watanabe A, Yamada M, Yasuda M, Tabata S (1996) Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. Strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res 3(3):109–136. doi:10.1093/dnares/3.3.109
Kim BH, Park HS, Kim HJ, Kim GT, Chang IS, Lee J, Phung NT (2004) Enrichment of microbial community generating electricity using a fuel-cell-type electrochemical cell. Appl Microbiol Biotechnol 63(6):672–681. doi:10.1007/s00253-003-1412-6
Kim K-Y, Chae K-J, Choi M-J, Ajayi FF, Jang A, Kim C-W, Kim IS (2011) Enhanced coulombic efficiency in glucose-fed microbial fuel cells by reducing metabolite electron losses using dual-anode electrodes. Bioresour Technol 102(5):4144–4149. doi:10.1016/j.biortech.2010.12.036
Kim N, Choi Y, Jung S, Kim S (2000) Development of microbial fuel cells using proteus vulgaris. Bull Korean Chem Soc 21(1):44–48
Kruk J, Jemioła-Rzemińska M, Strzałka K (2003) Cytochrome c is reduced mainly by plastoquinol and not by superoxide in thylakoid membranes at low and medium light intensities: its specific interaction with thylakoid membrane lipids. Biochem J 375(1):215–220. doi:10.1042/bj20021820
Kruse O, Rupprecht J, Mussgnug JH, Dismukes GC, Hankamer B (2005) Photosynthesis: a blueprint for solar energy capture and biohydrogen production technologies. Photochem Photobiol Sci 4(12):957–970. doi:10.1039/b506923h
Lawrence BA, Suarez C, DePina A, Click E, Kolodny NH, Allen MM (1998) Two internal pools of soluble polyphosphate in the cyanobacterium Synechocystis sp. strain PCC 6308: an in vivo 31P NMR spectroscopic study. Arch Microbiol 169(3):195–200. doi:10.1007/s002030050560
Loferer-Krossbacher M, Klima J, Psenner R (1998) Determination of bacterial cell dry mass by transmission electron microscopy and densitometric image analysis. Appl Environ Microbiol 64(2):688–694
Logan BE (2009) Exoelectrogenic bacteria that power microbial fuel cells. Nat Rev Microbiol 7(5):375–381
Logan BE, Hamelers B, Rozendal R, Schröder U, Keller J, Freguia S, Aelterman P, Verstraete W, Rabaey K (2006) Microbial fuel cells: methodology and technology. Environ Sci Technol 40(17):5181–5192
Lovley DR (2008) The microbe electric: conversion of organic matter to electricity. Curr Opin Biotechnol 19(6):564–571. doi:10.1016/j.copbio.2008.10.005
Madiraju KS, Lyew D, Kok R, Raghavan V (2012) Carbon neutral electricity production by Synechocystis sp. PCC6803 in a microbial fuel cell. Bioresour Technol 110:214–218. doi:10.1016/j.biortech.2012.01.065
Mahadevan R, Schilling CH (2003) The effects of alternate optimal solutions in constraint-based genome-scale metabolic models. Metab Eng 5(4):264–276. doi:10.1016/j.ymben.2003.09.002
Mao L, Verwoerd W (2013) Selection of organisms for systems biology study of microbial electricity generation: a review. Int J Energy Environ Eng 4(1):17. doi:10.1186/2251-6832-4-17
Mao L, Verwoerd WS Computational comparison of mediated MFC current generation capacity of Chlamydomonas reinhardtii in photosynthetic and respiratory growth modes (submitted)
Mao L, Verwoerd WS (2013) Model-driven elucidation of the inherent capacity of Geobacter sulfurreducens for electricity generation. J Biol Eng 7(1):14. doi:10.1186/1754-1611-7-14
McCormick AJ, Bombelli P, Scott AM, Philips AJ, Smith AG, Fisher AC, Howe CJ (2011) Photosynthetic biofilms in pure culture harness solar energy in a mediatorless bio-photovoltaic cell (BPV) system. Energy Environ Sci 4(11):4699–4709
McKinlay JB, Zeikus JG (2004) Extracellular iron reduction is mediated in part by neutral red and hydrogenase in Escherichia coli. Appl Environ Microbiol 70(6):3467–3474. doi:10.1128/AEM.70.6.3467-3474.2004
Nakamura Y, Kaneko T, Hirosawa M, Miyajima N, Tabata S (1998) CyanoBase, a www database containing the complete nucleotide sequence of the genome of Synechocystis sp. strain PCC6803. Nucleic Acids Res 26(1):63–67. doi:10.1093/nar/26.1.63
Navarro E, Montagud A, Fernández de Córdoba P, Urchueguía JF (2009) Metabolic flux analysis of the hydrogen production potential in Synechocystis sp. PCC6803. Int J Hydrogen Energy 34(21):8828–8838. doi:10.1016/j.ijhydene.2009.08.036
Okamoto A, Nakamura R, Hashimoto K (2011) In-vivo identification of direct electron transfer from Shewanella oneidensis MR-1 to electrodes via outer-membrane OmcA–MtrCAB protein complexes. Electrochim Acta 56(16):5526–5531. doi:10.1016/j.electacta.2011.03.076
Orth JD, Thiele I, Palsson BO (2010) What is flux balance analysis? Nat Biotechnol 28(3):245–248. URL http://www.nature.com/nbt/journal/v28/n3/abs/nbt.1614.html#supplementary-information
Park DH, Kim SK, Shin IH, Jeong YJ (2000) Electricity production in biofuel cell using modified graphite electrode with Neutral Red. Biotechnol Lett 22(16):1301–1304. doi:10.1023/a:1005674107841
Park DH, Zeikus JG (2000) Electricity generation in microbial fuel cells using neutral red as an electronophore. Appl Environ Microbiol 66(4):1292–1297. doi:10.1128/aem.66.4.1292-1297.2000
Pereyra V, Saunders M, Castillo J (2013) Equispaced Pareto front construction for constrained bi-objective optimization. Math Comput Model 57(9–10):2122–2131. doi:10.1016/j.mcm.2010.12.044
Pinchuk GE, Hill EA, Geydebrekht OV, De Ingeniis J, Zhang X, Osterman A, Scott JH, Reed SB, Romine MF, Konopka AE, Beliaev AS, Fredrickson JK, Reed JL (2010) Constraint-based model of Shewanella oneidensis MR-1 metabolism: a tool for data analysis and hypothesis generation. PLoS Comput Biol 6(6):e1000822. doi:10.1371/journal.pcbi.1000822
Pisciotta JM, Zou Y, Baskakov IV (2010) Light-dependent electrogenic activity of cyanobacteria. PLoS ONE 5(5):e10821. doi:10.1371/journal.pone.0010821
Pisciotta JM, Zou Y, Baskakov IV (2011) Role of the photosynthetic electron transfer chain in electrogenic activity of cyanobacteria. Appl Microbiol Biotechnol 91(2):377–385. doi:10.1007/s00253-011-3239-x
Rabaey K, Verstraete W (2005) Microbial fuel cells: novel biotechnology for energy generation. Trends Biotechnol 23(6):291–298. doi:10.1016/j.tibtech.2005.04.008
Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, Lovley DR (2005) Extracellular electron transfer via microbial nanowires. Nature 435(7045):1098–1101. doi:10.1038/nature03661
Reguera G, Nevin KP, Nicoll JS, Covalla SF, Woodard TL, Lovley DR (2006) Biofilm and nanowire production leads to increased current in Geobacter sulfurreducens fuel cells. Appl Environ Microbiol 72(11):7345–7348. doi:10.1128/AEM.01444-06
Rocha I, Maia P, Evangelista P, Vilaca P, Soares S, Pinto JP, Nielsen J, Patil KR, Ferreira EC, Rocha M (2010) OptFlux: an open-source software platform for in silico metabolic engineering. BMC Syst Biol 4:45. doi:10.1186/1752-0509-4-45
Rosenbaum M, Schröder U (2010) Photomicrobial solar and fuel cells. Electroanalysis 22(7–8):844–855. doi:10.1002/elan.200800005
Schellenberger J, Que R, Fleming RM, Thiele I, Orth JD, Feist AM, Zielinski DC, Bordbar A, Lewis NE, Rahmanian S, Kang J, Hyduke DR, Palsson BO (2011) Quantitative prediction of cellular metabolism with constraint-based models: the COBRA Toolbox v2.0. Nat Protoc 6(9):1290–1307. doi:10.1038/nprot.2011.308
Schröder U (2007) Anodic electron transfer mechanisms in microbial fuel cells and their energy efficiency. Phys Chem Chem Phys 9(21):2619–2629
Sharma V, Kundu PP (2010) Biocatalysts in microbial fuel cells. Enzyme Microb Technol 47(5):179–188. doi:10.1016/j.enzmictec.2010.07.001
Shastri AA, Morgan JA (2005) Flux balance analysis of photoautotrophic metabolism. Biotechnol Prog 21(6):1617–1626. doi:10.1021/bp050246d
Shukla AK, Suresh P, Berchmans S, Rajendran A (2004) Biological fuel cells and their applications. Curr Sci 87(4):455–468
Song H-S, Ramkrishna D, Pinchuk GE, Beliaev AS, Konopka AE, Fredrickson JK (2013) Dynamic modeling of aerobic growth of Shewanella oneidensis. Predicting triauxic growth, flux distributions, and energy requirement for growth. Metab Eng 15(0):25–33. doi:10.1016/j.ymben.2012.08.004
Steuer R, Knoop H, Machné R (2012) Modelling cyanobacteria: from metabolism to integrative models of phototrophic growth. J Exp Bot 63(6):2259–2274. doi:10.1093/jxb/ers018
Sund CJ, McMasters S, Crittenden SR, Harrell LE, Sumner JJ (2007) Effect of electron mediators on current generation and fermentation in a microbial fuel cell. Appl Microbiol Biotechnol 76(3):561–568. doi:10.1007/s00253-007-1038-1
Takahashi H, Uchimiya H, Hihara Y (2008) Difference in metabolite levels between photoautotrophic and photomixotrophic cultures of Synechocystis sp. PCC 6803 examined by capillary electrophoresis electrospray ionization mass spectrometry. J Exp Bot 59(11):3009–3018. doi:10.1093/jxb/ern157
Tanaka K, Kashiwagi N, Ogawa T (1988) Effects of light on the electrical output of bioelectrochemical fuel-cells containing Anabaena variabilis M-2: mechanism of the post-illumination burst. J Chem Technol Biotechnol 42(3):235–240. doi:10.1002/jctb.280420307
van Hoek M, Merks R (2012) Redox balance is key to explaining full vs. partial switching to low-yield metabolism. BMC Syst Biol 6(1):22
Varma A, Palsson BO (1994) Metabolic flux balancing: basic concepts, scientific and practical use. Nat Biotechnol 12(10):994–998
Vermaas WFJ (2001) Photosynthesis and respiration in cyanobacteria. In: eLS. Wiley, London. doi:10.1038/npg.els.0001670
Verwoerd W (2011) A new computational method to split large biochemical networks into coherent subnets. BMC Syst Biol 5(1):25
Verwoerd W (2012) Interactive extraction of metabolic subnets—the Netsplitter software implementation. J Mol Eng Syst Biol 1(1):2–2
Virdis B, Freguia S, Rozendal RA, Rabaey K, Yuan Z, Keller J (2011) 4.18—microbial fuel cells. In: Editor-in-Chief: Peter W (ed) Treatise on water science. Elsevier, Oxford, pp 641–665. doi:10.1016/b978-0-444-53199-5.00098-1
Wang K, Liu Y, Chen S (2011) Improved microbial electrocatalysis with neutral red immobilized electrode. J Power Sour 196(1):164–168. doi:10.1016/j.jpowsour.2010.06.056
Whitman WB, Coleman DC, Wiebe WJ (1998) Prokaryotes: the unseen majority. Proc Natl Acad Sci 95(12):6578–6583
Wilkinson S (2000) “Gastrobots”—benefits and challenges of microbial fuel cells in foodpowered robot applications. Auton Robots 9(2):99–111. doi:10.1023/a:1008984516499
Yagishita T, Horigome T, Tanaka K (1993) Effects of light, CO2 and inhibitors on the current output of biofuel cells containing the photosynthetic organism Synechococcus sp. J Chem Technol Biotechnol 56(4):393–399. doi:10.1002/jctb.280560411
Yang C, Hua Q, Shimizu K (2002) Metabolic flux analysis in Synechocystis using isotope distribution from 13C-labeled glucose. Metab Eng 4(3):202–216. doi:10.1006/mben.2002.0226
Yang Y, Xu M, Guo J, Sun G (2012) Bacterial extracellular electron transfer in bioelectrochemical systems. Process Biochem 47(12):1707–1714. doi:10.1016/j.procbio.2012.07.032
Yoshikawa K, Kojima Y, Nakajima T, Furusawa C, Hirasawa T, Shimizu H (2011) Reconstruction and verification of a genome-scale metabolic model for Synechocystis sp. PCC6803. Appl Microbiol Biotechnol 92 (2):347–358. doi:10.1007/s00253-011-3559-x
Zhao F, Harnisch F, Schröder U, Scholz F, Bogdanoff P, Herrmann I (2006) Challenges and constraints of using oxygen cathodes in microbial fuel cells. Environ Sci Technol 40(17):5193–5199. doi:10.1021/es060332p
Zou Y, Pisciotta J, Billmyre RB, Baskakov IV (2009) Photosynthetic microbial fuel cells with positive light response. Biotechnol Bioeng 104(5):939–946. doi:10.1002/bit.22466
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mao, L., Verwoerd, W.S. Genome-scale stoichiometry analysis to elucidate the innate capability of the cyanobacterium Synechocystis for electricity generation. J Ind Microbiol Biotechnol 40, 1161–1180 (2013). https://doi.org/10.1007/s10295-013-1308-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-013-1308-0