Abstract
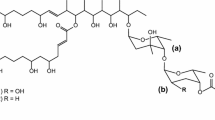
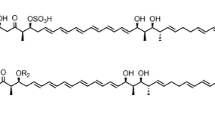
Some polyketide-derived bioactive compounds contain sugars attached to the aglycone core, and these sugars often enhance or impart specific biological activity to the molecule. Mycinamicin II, a 16-member macrolide antibiotic produced by Micromonospora griseorubida A11725, contains a branched lactone and two different deoxyhexose sugars, d-desosamine and d-mycinose, at the C-5 and C-21 positions, respectively. We previously engineered an expression plasmid pSETmycinose containing the d-mycinose biosynthesis genes from M. griseorubida A11725. This plasmid was introduced into Micromonospora sp. FERM BP-1076 cells, which produce the 16-membered macrolide antibiotic izenamicin. The resulting engineered strain TPMA0041 produced 23-O-mycinosyl-20-deoxy-izenamicin B1 and 22-O-mycinosyl-izenamicin B2. 23-O-mycinosyl-20-deoxy-izenamicin B1 has been produced by the engineered strain M. rosaria TPMA0001 containing pSETmycinose as 23-O-mycinosyl-20-deoxo-20-dihydro-12,13-deepoxyrosamicin (=IZI) in our recent study, and 22-O-mycinosyl-izenamicin B2 has previously been synthesized as a macrolide antibiotic TMC-016 with strong antibacterial activity. The production of 22-O-mycinosyl-izenamicin B2 (=TMC-016) was increased when propionate, a precursor of methylmalonyl-CoA, was added to the culture broth.



Similar content being viewed by others
References
Anzai Y, Iizaka Y, Li W, Idemoto N, Tsukada S, Koike K, Kinoshita K, Kato F (2009) Production of rosamicin derivatives in Micromonospora rosaria by introduction of D-mycinose biosynthetic gene with ϕC31-derived integration vector pSET152. J Ind Microbiol Biotechnol 36:1013–1021
Anzai Y, Ishii Y, Yoda Y, Kinoshita K, Kato F (2004) The targeted inactivation of polyketide synthase mycAV in the mycinamicin producer, Micromonospora griseorubida, and a complementation study. FEMS Microbiol Lett 238:315–320
Anzai Y, Saito N, Tanaka M, Kinoshita K, Koyama Y, Kato F (2003) Organization of the biosynthetic gene cluster for the polyketide macrolide mycinamicin in Micromonospora griseorubida. FEMS Microbiol Lett 218:135–141
Anzai Y, Sakai A, Li W, Iizaka Y, Koike K, Kinoshita K, Kato F (2010) Isolation and characterization of 23-O-mycinosyl-20-dihydro-rosamicin: a new rosamicin analogue derived from engineered Micromonospora rosaria. J Antibiot 63:325–328
Baltz RH (2012) Streptomyces temperate bacteriophage integration systems for stable genetic engineering of actinomycetes (and other organisms). J Ind Microbiol Biotechnol 39:661–672
Choi SU, Lee CK, Hwang YI, Kinoshita H, Nihira T (2004) Intergeneric conjugal transfer of plasmid DNA from Escherichia coli to Kitasatospora setae, a bafilomycin B1 producer. Arch Microbiol 181:294–298
Combes P, Till R, Bee S, Smith MC (2002) The Streptomyces genome contains multiple pseudo-attB sites for the ϕC31-encoded site-specific recombination system. J Bacteriol 184:5746–5752
Fujiwara T, Watanabe H, Kogami Y, Shiritani Y, Sakakibara H (1989) 19-Deformyl-4′-deoxydesmycosin (TMC-016): synthesis and biological properties of a unique 16-membered macrolide antibiotic. J Antibiot 42:903–912
Ha HS, Hwang YI, Choi SU (2008) Application of conjugation using ϕC31 att/int system for Actinoplanes teichomyceticus, a producer of teicoplanin. Biotechnol Lett 30:1233–1238
Han SJ, Park SW, Park BW, Sim SJ (2008) Selective production of epothilone B by heterologous expression of propionyl-CoA synthetase in Sorangium cellulosum. J Microbiol Biotechnol 18:135–137
Hatano K, Higashide E, Shibata M (1976) Studies on juvenimicin, a new antibiotic. I. Taxonomy, fermentation and antimicrobial properties. J Antibiot 29:1163–1170
Hopwood DA (1997) Genetic contributions to understanding polyketide synthases. Chem Rev 97:2465–2498
Imai H, Suzuki K, Morioka M, Sasaki T, Tanaka K, Kadota S, Iwanami M, Saito T, Eiki H (1989) Izenamicins: macrolide antibiotics. J Antibiot 42:1000–1002
Japan Society of Chemotherapy (1990) Method of MIC determination by broth microdilution method. Chemotherapy (Tokyo) 38:102–105. In Japanese
Kinoshita K, Imura Y, Takenaka S, Hayashi M (1989) Mycinamicins, new macrolide antibiotics. XI. Isolation and structure elucidation of a key intermediate in the biosynthesis of the mycinamicins, mycinamicin VIII. J Antibiot 42:1869–1872
Kishi T, Harada S, Yamana H, Miyake A (1976) Studies on juvenimicin, a new antibiotic. II. Isolation, chemical characterization and structures. J Antibiot 29:1171–1181
Kuhstoss S, Rao RN (1991) Analysis of the integration function of the streptomycete bacteriophage ϕC31. J Mol Biol 222:897–908
Lee BK, Puar MS, Patel M, Bartner P, Lotvin J, Munayyer H, Waitz JA (1983) Multistep bioconversion of 20-deoxo-20-dihydro-12,13-deepoxy-12,13-dehydrorosaranolide to 22-hydroxy-23-O-mycinosyl-20-deoxo-20-dihydro-12,13-deepoxy-rosaramicin. J Antibiot 36:742–744
Mo S, Ban YH, Park JW, Yoo YJ, Yoon YJ (2009) Enhanced FK506 production in Streptomyces clavuligerus CKD1119 by engineering the supply of methylmalonyl-CoA precursor. J Ind Microbiol Biotechnol 36:1473–1482
Murli S, Kennedy J, Dayem LC, Carney JR, Kealey JT (2003) Metabolic engineering of Escherichia coli for improved 6-deoxyerythronolide B production. J Ind Microbiol Biotechnol 30:500–509
Park SR, Han AR, Ban YH, Yoo YJ, Kim EJ, Yoon YJ (2010) Genetic engineering of macrolide biosynthesis: past advances, current state, and future prospects. Appl Microbiol Biotechnol 85:1227–1239
Rheims H, Schumann P, Rohde M, Stackebrandt E (1998) Verrucosispora gifhornensis gen. nov., sp. nov., a new member of the actinobacterial family Micromonosporaceae. Int J Syst Bacteriol 48(Pt 4):1119–1127
Rodriguez E, McDaniel R (2001) Combinatorial biosynthesis of antimicrobials and other natural products. Curr Opin Microbiol 4:526–534
Salas JA, Méndez C (2007) Engineering the glycosylation of natural products in actinomycetes. Trends Microbiol 15:219–232
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Sioud S, Aigle B, Karray-Rebai I, Smaoui S, Bejar S, Mellouli L (2009) Integrative gene cloning and expression system for Streptomyces sp. US 24 and Streptomyces sp. TN 58 bioactive molecule producing strains. J Biomed Biotechnol 2009:464986
Tsukada S, Anzai Y, Li S, Kinoshita K, Sherman DH, Kato F (2010) Gene targeting for O-methyltransferase genes, mycE and mycF, on the chromosome of Micromonospora griseorubida producing mycinamicin with a disruption cassette containing the bacteriophage ϕC31 attB attachment site. FEMS Microbiol Lett 304:148–156
Acknowledgments
We thank Dr. Shingo Fujisaki (Toho University) for help with LC–MS analysis, Dr. Wei Li (Toho University) for help with NMR analysis, and Ms. Yumi Ichikawa (Toho University) for help with antibacterial activity assay.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sakai, A., Mitsumori, A., Furukawa, M. et al. Production of a hybrid 16-membered macrolide antibiotic by genetic engineering of Micromonospora sp. TPMA0041. J Ind Microbiol Biotechnol 39, 1693–1701 (2012). https://doi.org/10.1007/s10295-012-1173-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-012-1173-2