Abstract
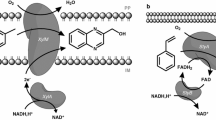
Selection of the ideal microbe is crucial for whole-cell biotransformations, especially if the target reaction intensively interacts with host cell functions. Asymmetric styrene epoxidation is an example of a reaction which is strongly dependent on the host cell owing to its requirement for efficient cofactor regeneration and stable expression of the styrene monooxygenase genes styAB. On the other hand, styrene epoxidation affects the whole-cell biocatalyst, because it involves toxic substrate and products besides the burden of additional (recombinant) enzyme synthesis. With the aim to compare two fundamentally different strain engineering strategies, asymmetric styrene epoxidation by StyAB was investigated using the engineered wild-type strain Pseudomonas sp. strain VLB120ΔC, a styrene oxide isomerase (StyC) knockout strain able to accumulate (S)-styrene oxide, and recombinant E. coli JM101 carrying styAB on the plasmid pSPZ10. Their performance was analyzed during fed-batch cultivation in two-liquid phase biotransformations with respect to specific activity, volumetric productivity, product titer, tolerance of toxic substrate and products, by-product formation, and product yield on glucose. Thereby, Pseudomonas sp. strain VLB120ΔC proved its great potential by tolerating high styrene oxide concentrations and by the absence of by-product formation. The E. coli-based catalyst, however, showed higher specific activities and better yields on glucose. The results not only show the importance but also the complexity of host cell selection and engineering. Finding the optimal strain engineering strategy requires profound understanding of bioprocess and biocatalyst operation. In this respect, a possible negative influence of solvent tolerance on yield and activity is discussed.



Similar content being viewed by others
References
Axe DD, Bailey JE (1995) Transport of lactate and acetate through the energized cytoplasmic membrane of E. coli. Biotechnol Bioeng 47(1):8–19
Beltrametti F, Marconi AM, Bestetti G, Colombo C, Galli E, Ruzzi M, Zennaro E (1997) Sequencing and functional analysis of styrene catabolism genes from Pseudomonas fluorescens ST. Appl Environ Microbiol 63(6):2232–2239
Bertani G (1951) Studies on lysogenesis I. The mode of phage liberation by lysogenic E. coli. J Bacteriol 62(3):293–300
Blank LM, Ebert BE, Bühler B, Schmid A (2008) Metabolic capacity estimation of E. coli as platform for redox biocatalysis: constraint based modeling and experimental verification. Biotechnol Bioeng 100(6):1050–1065
Blank LM, Ebert BE, Bühler K, Bühler B (2010) Redox biocatalysis and metabolism: molecular mechanisms and metabolic network analysis. Antioxid Redox Signal 13(3):349–394
Blank LM, Ionidis G, Ebert BE, Bühler B, Schmid A (2008) Metabolic response of P. putida during redox biocatalysis in the presence of a second octanol phase. FEBS J 275(20):5173–5190
Bühler B, Bollhalder I, Hauer B, Witholt B, Schmid A (2003) Use of the two-liquid phase concept to exploit kinetically controlled multistep biocatalysis. Biotechnol Bioeng 81(6):683–694
Bühler B, Schmid A (2004) Process implementation aspects for biocatalytic hydrocarbon oxyfunctionalization. J Biotechnol 113(1–3):183–210
Bühler B, Schmid A, Hauer B, Witholt B (2000) Xylene monooxygenase catalyzes the multistep oxygenation of toluene and pseudocumene to corresponding alcohols, aldehydes, and acids in E. coli JM101. J Biol Chem 275(14):10085–10092
Bühler B, Straathof AJJ, Witholt B, Schmid A (2006) Analysis of two-liquid-phase multistep biooxidation based on a process model: indications for biological energy shortage. Org Process Res Dev 10(3):628–643
Bylund F, Collet E, Enfors SO, Larsson G (1998) Substrate gradient formation in the large-scale bioreactor lowers cell yield and increases by-product formation. Bioprocess Eng 18(3):171–180
Cruden DL, Wolfram JH, Rogers RD, Gibson DT (1992) Physiological properties of a Pseudomonas strain which grows with p-xylene in a two-phase (organic-aqueous) medium. Appl Environ Microbiol 58(9):2723–2729
de Bont JAM (1998) Solvent-tolerant bacteria in biocatalysis. Trends Biotechnol 16(12):493–499
Demain AL (2000) Microbial biotechnology. Trends Biotechnol 18(1):26–31
Duetz WA, van Beilen JB, Witholt B (2001) Using proteins in their natural environment: potential and limitations of microbial whole-cell hydroxylations in applied biocatalysis. Curr Opin Biotechnol 12(4):419–425
Enfors SO, Jahic M, Rozkov A, Xu B, Hecker M, Jürgen B, Krüger E, Schweder T, Hamer G, O’Beirne D, Noisommit-Rizzi N, Reuss M, Boone L, Hewitt C, McFarlane C, Nienow A, Kovacs T, Trägardh C, Fuchs L, Revstedt J, Friberg PC, Hjertager B, Blomsten G, Skogman H, Hjort S, Hoeks F, Lin HY, Neubauer P, van der Lans R, Luyben K, Vrabel P, Manelius A (2001) Physiological responses to mixing in large scale bioreactors. J Biotechnol 85(2):175–185
Halan B, Schmid A, Buehler K (2011) Real-time solvent tolerance analysis of Pseudomonas sp. strain VLB120ΔC catalytic biofilms. Appl Environ Microbiol 77(5):1563–1571
Heijnen JJ, van Dijken JP (1992) In search of a thermodynamic description of biomass yields for the chemotropic growth of microorganisms. Biotechnol Bioeng 39(8):833–858
Heipieper HJ, Neumann G, Cornelissen S, Meinhardt F (2007) Solvent-tolerant bacteria for biotransformations in two-phase fermentation systems. Appl Microbiol Biotechnol 74(5):961–973
Hofstetter K, Lutz J, Lang I, Witholt B, Schmid A (2004) Coupling of biocatalytic asymmetric epoxidation with NADH regeneration in organic-aqueous emulsions. Angew Chem Int Ed 43(16):2163–2166
Inoue A, Horikoshi K (1989) A Pseudomonas thrives in high concentrations of toluene. Nature 338(6212):264–266
Isken S, de Bont JAM (1998) Bacteria tolerant to organic solvents. Extremophiles 2(3):229–238
Isken S, Derks A, Wolffs PF, de Bont JA (1999) Effect of organic solvents on the yield of solvent-tolerant P. putida S12. Appl Environ Microbiol 65(6):2631–2635
Kuhn D, Blank LM, Schmid A, Bühler B (2010) Systems biotechnology: rational whole-cell biocatalyst and bioprocess design. Eng Life Sci 10(5):384–397
Kuhn D, Julsing MK, Heinzle E, Bühler B (2012) Systematic optimization of a biocatalytic two-liquid phase oxyfunctionalization process guided by ecological and economic assessment. Green Chem 14:645–653
Kuhn D, Kholiq MA, Heinzle E, Bühler B, Schmid A (2010) Intensification and economic and ecological assessment of a biocatalytic oxyfunctionalization process. Green Chem 12(5):815–827
Leak DJ, Sheldon RA, Woodley JM, Adlercreutz P (2009) Biocatalysts for selective introduction of oxygen. Biocatal Biotransform 27(1):1–26
Maruyama T, Iida H, Kakidani H (2003) Oxidation of both termini of p- and m-xylene by E. coli transformed with xylene monooxygenase gene. J Mol Catal B Enzym 21(4–6):211–219
Messing J (1979) A multi-purpose cloning system based on the single stranded bacteriophage M13. Recomb DNA Tech Bull 79–99(2):43–48
Meyer D, Bühler B, Schmid A (2006) Process and catalyst design objectives for specific redox biocatalysis. Adv Appl Microbiol 59:53–91
Meyer D, Witholt B, Schmid A (2005) Suitability of recombinant E. coli and P. putida strains for selective biotransformation of m-nitrotoluene by xylene monooxygenase. Appl Environ Microbiol 71(11):6624–6632
Otero JM, Nielsen J (2010) Industrial systems biology. Biotechnol Bioeng 105(3):439–460
Panke S, Held M, Wubbolts MG, Witholt B, Schmid A (2002) Pilot-scale production of (S)-styrene oxide from styrene by recombinant E. coli synthesizing styrene monooxygenase. Biotechnol Bioeng 80(1):33–41
Panke S, Meyer A, Huber CM, Witholt B, Wubbolts MG (1999) An alkane-responsive expression system for the production of fine chemicals. Appl Environ Microbiol 65(6):2324–2332
Panke S, Witholt B, Schmid A, Wubbolts MG (1998) Towards a biocatalyst for (S)-styrene oxide production: characterization of the styrene degradation pathway of Pseudomonas sp. strain VLB120. Appl Environ Microbiol 64(9):2032–2043
Panke S, Wubbolts MG, Schmid A, Witholt B (2000) Production of enantiopure styrene oxide by recombinant E. coli synthesizing a two-component styrene monooxygenase. Biotechnol Bioeng 69(1):91–100
Park JB, Bühler B, Habicher T, Hauer B, Panke S, Witholt B, Schmid A (2006) The efficiency of recombinant E. coli as biocatalyst for stereospecific epoxidation. Biotechnol Bioeng 95(3):501–512
Park JB, Bühler B, Panke S, Witholt B, Schmid A (2007) Carbon metabolism and product inhibition determine the epoxidation efficiency of solvent-tolerant Pseudomonas sp. strain VLB120C. Biotechnol Bioeng 98(6):1219–1229
Park JH, Lee SY, Kim TY, Kim HU (2008) Application of systems biology for bioprocess development. Trends Biotechnol 26(8):404–412
Ramos JL, Duque E, Huertas MJ, Haidour A (1995) Isolation and expansion of the catabolic potential of a P. putida strain able to grow in the presence of high concentrations of aromatic hydrocarbons. J Bacteriol 177(14):3911–3916
Rothen SA, Sauer M, Sonnleitner B, Witholt B (1998) Biotransformation of octane by E. coli HB101[pGEc47] on defined medium: octanoate production and product inhibition. Biotechnol Bioeng 58(4):356–365
Ruinatscha R, Dusny C, Bühler K, Schmid A (2009) Productive asymmetric styrene epoxidation based on a next generation electroenzymatic methodology. Adv Synth Catal 351(14–15):2505–2515
Sambrook J, Russell DW (2001) Molecular cloning–a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York, NY, USA
Santos PM, Blatny JM, Di Bartolo I, Valla S, Zennaro E (2000) Physiological analysis of the expression of the styrene degradation gene cluster in Pseudomonas fluorescens ST. Appl Environ Microbiol 66(4):1305–1310
Schmid A, Dordick JS, Hauer B, Kiener A, Wubbolts M, Witholt B (2001) Industrial biocatalysis today and tomorrow. Nature 409(6817):258–268
Segura A, Godoy P, van Dillewijn P, Hurtado A, Arroyo N, Santacruz S, Ramos JL (2005) Proteomic analysis reveals the participation of energy- and stress-related proteins in the response of P. putida DOT-T1E to toluene. J Bacteriol 187(17):5937–5945
van Beilen JB, Duetz WA, Schmid A, Witholt B (2003) Practical issues in the application of oxygenases. Trends Biotechnol 21(4):170–177
Verduyn C, Stouthamer AH, Scheffers WA, Vandijken JP (1991) A theoretical evaluation of growth yields of yeasts. Anton Leeuw Int J G 59(1):49–63
Volkers RJM, de Jong AL, Hulst AG, van Baar BLM, de Bont JAM, Wery J (2006) Chemostat-based proteomic analysis of toluene-affected P. putida S12. Environ Microbiol 8(9):1674–1679
von Stockar U, Maskow T, Liu JS, Marison IW, Patino R (2006) Thermodynamics of microbial growth and metabolism: an analysis of the current situation. J Biotechnol 121(4):517–533
Walton AZ, Stewart JD (2004) Understanding and improving NADPH-dependent reactions by nongrowing E. coli cells. Biotechnol Progr 20(2):403–411
Weber FJ, Ooijkaas LP, Schemen RMW, Hartmans S, Debont JAM (1993) Adaptation of P. putida S12 to high concentrations of styrene and other organic solvents. Appl Environ Microbiol 59(10):3502–3504
Acknowledgments
This work was supported by the Ministry of Innovation, Science, Research and Technology of North Rhine-Westphalia (Bio.NRW, Technology Platform Biocatalysis, RedoxCell).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kuhn, D., Bühler, B. & Schmid, A. Production host selection for asymmetric styrene epoxidation: Escherichia coli vs. solvent-tolerant Pseudomonas . J Ind Microbiol Biotechnol 39, 1125–1133 (2012). https://doi.org/10.1007/s10295-012-1126-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-012-1126-9