Abstract
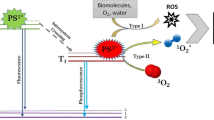
The goal of this study was to investigate the photodynamic toxicity of TMPyP (5, 10, 15, 20-Tetrakis (1-methylpyridinium-4-yl)-porphyrin tetra p-toluenesulfonate) in combination with short pulses (ms) of an intense pulse light source within 10 s against Bacillus atrophaeus, Staphylococcus aureus, Methicillin-resistant S. aureus and Escherichia coli, major pathogens in food industry and in health care, respectively. Bacteria were incubated with a photoactive dye (TMPyP) that is subsequently irradiated with visible light flashes of 100 ms to induce oxidative damage immediately by generation of reactive oxygen species like singlet oxygen. A photodynamic killing efficacy of up to 6 log10 (>99.9999%) was achieved within a total treatment time of 10 s using a concentration range of 1–100 μmol TMPyP and multiple light flashes of 100 ms (from 20 J cm−2 up to 80 J cm−2). Both incubation of bacteria with TMPyP alone or application of light flashes only did not have any negative effect on bacteria survival. Here we could demonstrate for the first time that the combination of TMPyP as the respective photosensitizer and a light flash of 100 ms of an intense pulsed light source is enough to generate sufficient amounts of reactive oxygen species to kill these pathogens within a few seconds. Increasing antibiotic resistance requires fast and efficient new approaches to kill bacteria, therefore the photodynamic process seems to be a promising tool for disinfection of horizontal surfaces in industry and clinical purposes where savings in time is a critical point to achieve efficient inactivation of microorganisms.





Similar content being viewed by others
References
al-Masaudi SB, Day MJ, Russell AD (1991) Antimicrobial resistance and gene transfer in Staphylococcus aureus. J Appl Bacteriol 70(4):279–290
Alves E, Costa L, Carvalho CM, Tome JP, Faustino MA, Neves MG, Tome AC, Cavaleiro JA, Cunha A, Almeida A (2009) Charge effect on the photoinactivation of Gram-negative and Gram-positive bacteria by cationic meso-substituted porphyrins. BMC Microbiol 9:70
Ang JY, Ezike E, Asmar BI (2004) Antibacterial resistance. Indian J Pediatr 71(3):229–239
Appelbaum PC (2006) MRSA–the tip of the iceberg. Clin Microbiol Infect 12(Suppl 2):3–10
Babilas P, Schreml S, Szeimies RM, Landthaler M (2010) Intense pulsed light (IPL): a review. Lasers Surg Med 42(2):93–104
Baquero F, Negri MC, Morosini MI, Blazquez J (1998) Antibiotic-selective environments. Clin Infect Dis 27(Suppl 1):S5–S11
Birosova L, Mikulasova M (2009) Development of triclosan and antibiotic resistance in Salmonella enterica serovar Typhimurium. J Med Microbiol 58(Pt 4):436–441
Blot S, Depuydt P, Vandewoude K, De Bacquer D (2007) Measuring the impact of multidrug resistance in nosocomial infection. Curr Opin Infect Dis 20(4):391–396
Bower CK, Daeschel MA (1999) Resistance responses of microorganisms in food environments. Int J Food Microbiol 50(1–2):33–44
Boyce JM, Pittet D (2002) Guideline for hand hygiene in health-care settings. recommendations of the healthcare infection control practices advisory committee and the HIPAC/SHEA/APIC/IDSA hand hygiene task force. Am J Infect Control 30(8):S1–S46
Brancaleon L, Moseley H (2002) Laser and non-laser light sources for photodynamic therapy. Lasers Med Sci 17(3):173–186
Branski LK, Al-Mousawi A, Rivero H, Jeschke MG, Sanford AP, Herndon DN (2009) Emerging infections in burns. Surg Infect (Larchmt) 10(5):389–397
Breitenbach T, Kuimova MK, Gbur P, Hatz S, Schack NB, Pedersen BW, Lambert JD, Poulsen L, Ogilby PR (2009) Photosensitized production of singlet oxygen: spatially resolved optical studies in single cells. Photochem Photobiol Sci 8(4):442–452
Chapple RM, Inglis B, Stewart PR (1992) Lethal and mutational effects of solar and UV radiation on Staphylococcus aureus. Arch Microbiol 157(3):242–248
Exon JH (1984) A review of chlorinated phenols. Vet Hum Toxicol 26(6):508–520
Feese E, Ghiladi RA (2009) Highly efficient in vitro photodynamic inactivation of Mycobacterium smegmatis. J Antimicrob Chemother 64(4):782–785
Gad F, Zahra T, Francis KP, Hasan T, Hamblin MR (2004) Targeted photodynamic therapy of established soft-tissue infections in mice. Photochem Photobiol Sci 3(5):451–458
Garcez AS, Nunez SC, Hamblin MR, Ribeiro MS (2008) Antimicrobial effects of photodynamic therapy on patients with necrotic pulps and periapical lesion. J Endod 34(2):138–142
Garcez AS, Ribeiro MS, Tegos GP, Nunez SC, Jorge AO, Hamblin MR (2007) Antimicrobial photodynamic therapy combined with conventional endodontic treatment to eliminate root canal biofilm infection. Lasers Surg Med 39(1):59–66
Goldman MP, Weiss RA, Weiss MA (2005) Intense pulsed light as a nonablative approach to photoaging. Dermatol Surg 31(2):1179–1187 discussion 1187
Gottfried V, Peled D, Winkelman JW, Kimel S (1988) Photosensitizers in organized media: singlet oxygen production and spectral properties. Photochem Photobiol 48(2):157–163
Grinholc M, Szramka B, Kurlenda J, Graczyk A, Bielawski KP (2008) Bactericidal effect of photodynamic inactivation against methicillin-resistant and methicillin-susceptible Staphylococcus aureus is strain-dependent. J Photochem Photobiol B 90(1):57–63
Grinholc M, Szramka B, Olender K, Graczyk A (2007) Bactericidal effect of photodynamic therapy against methicillin-resistant Staphylococcus aureus strain with the use of various porphyrin photosensitizers. Acta Biochim Pol 54(3):665–670
Grinholc M, Zawacka-Pankau J, Gwizdek-Wisniewska A, Bielawski KP (2011) Evaluation of the role of the pharmacological inhibition of Staphylococcus aureus multidrug resistance pumps and the variable levels of the uptake of the sensitizer in the strain-dependent response of Staphylococcus aureus to PPArg(2)-based photodynamic inactivation. Photochem Photobiol 86(5):1118–1126
Huycke MM, Sahm DF, Gilmore MS (1998) Multiple-drug resistant enterococci: the nature of the problem and an agenda for the future. Emerg Infect Dis 4(2):239–249
Imlay JA, Linn S (1988) DNA damage and oxygen radical toxicity. Science 240(4857):1302–1309
Jori G, Fabris C, Soncin M, Ferro S, Coppellotti O, Dei D, Fantetti L, Chiti G, Roncucci G (2006) Photodynamic therapy in the treatment of microbial infections: basic principles and perspective applications. Lasers Surg Med 38(5):468–481
Komagoe K, Kato H, Inoue T, Katsu T (2011) Continuous real-time monitoring of cationic porphyrin-induced photodynamic inactivation of bacterial membrane functions using electrochemical sensors. Photochem Photobiol Sci 10(7):1181–1188
Komerik N, Nakanishi H, MacRobert AJ, Henderson B, Speight P, Wilson M (2003) In vivo killing of porphyromonas gingivalis by toluidine blue-mediated photosensitization in an animal model. Antimicrob Agents Chemother 47(3):932–940
Maisch T, Baier J, Franz B, Maier M, Landthaler M, Szeimies RM, Baumler W (2007) The role of singlet oxygen and oxygen concentration in photodynamic inactivation of bacteria. Proc Natl Acad Sci USA 104(17):7223–7228
Maisch T, Moor AC, Regensburger J, Ortland C, Szeimies RM, Baumler W (2011) Intense pulse light and 5-ALA PDT: phototoxic effects in vitro depend on the spectral overlap with protoporphyrine IX but do not match cut-off filter notations. Lasers Surg Med 43(2):176–182
Maisch T, Wagner J, Papastamou V, Nerl HJ, Hiller KA, Szeimies RM, Schmalz G (2009) Combination of 10% EDTA, photosan, and a blue light hand-held photopolymerizer to inactivate leading oral bacteria in dentistry in vitro. J Appl Microbiol 107(5):1569–1578
Mathur S, Singh R (2005) Antibiotic resistance in food lactic acid bacteria–a review. Int J Food Microbiol 105(3):281–295
Merchat M, Bertolini G, Giacomini P, Villanueva A, Jori G (1996) Meso-substituted cationic porphyrins as efficient photosensitizers of Gram-positive and Gram-negative bacteria. J Photochem Photobiol B 32(3):153–157
Miles AA, Misra SS, Irwin JO (1938) The estimation of the bactericidal power of the blood. J Hyg (Lond) 38(6):732–749
Nitzan Y, Dror R, Ladan H, Malik Z, Kimel S, Gottfried V (1995) Structure-activity relationship of porphines for photoinactivation of bacteria. Photochem Photobiol 62(2):342–347
Pottier R, Truscott TG (1986) The photochemistry of haematoporphyrin and related systems. Int J Radiat Biol Relat Stud Phys Chem Med 50(3):421–452
Ragas X, Dai T, Tegos GP, Agut M, Nonell S, Hamblin MR (2011) Photodynamic inactivation of Acinetobacter baumannii using phenothiazinium dyes: in vitro and in vivo studies. Lasers Surg Med 42(5):384–390
Raulin C, Greve B, Grema H (2003) IPL technology: a review. Lasers Surg Med 32(2):78–87
Salmon-Divon M, Nitzan Y, Malik Z (2004) Mechanistic aspects of Escherichia coli photodynamic inactivation by cationic tetra-meso(N-methylpyridyl)porphine. Photochem Photobiol Sci 3(5):423–429
Shamban AT (2009) Current and new treatments of photodamaged skin. Facial Plast Surg 25(5):337–346
Silvestry-Rodriguez N, Sicairos-Ruelas EE, Gerba CP, Bright KR (2007) Silver as a disinfectant. Rev Environ Contam Toxicol 191:23–45
Snellings WM, Weil CS, Maronpot RR (1984) A two-year inhalation study of the carcinogenic potential of ethylene oxide in Fischer 344 rats. Toxicol Appl Pharmacol 75(1):105–117
Usui Y (1973) Determination of quantum yield of singlet oxygen formation by photosensitization. Chem Lett 7:743–744
Wigle DT, Arbuckle TE, Walker M, Wade MG, Liu S, Krewski D (2007) Environmental hazards: evidence for effects on child health. J Toxicol Environ Health B Crit Rev 10(1–2):3–39
Wilkinson F, Helman W, Ross AB (1993) Quantum yields for the photosensitized formation of the lowest electronically excited singelt state of molecular oxygen in solution. J Phys Chem Ref Data 22:113–262
Acknowledgments
The excellent technical assistance of Ewa Kowalewski and Francesco Santarelli is gratefully acknowledged. No financial conflict of interest is declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maisch, T., Spannberger, F., Regensburger, J. et al. Fast and effective: intense pulse light photodynamic inactivation of bacteria. J Ind Microbiol Biotechnol 39, 1013–1021 (2012). https://doi.org/10.1007/s10295-012-1103-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-012-1103-3