Abstract
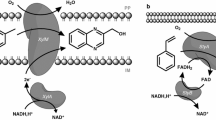
Carbazole 1,9a-dioxygenase (CarA), the first enzyme in the carbazole degradation pathway used by Pseudomonas sp., was expressed in E. coli under different conditions defined by experimental design. This enzyme depends on the coexistence of three components containing [2Fe–2S] clusters: CarAa, CarAc, and CarAd. The catalytic site is present in CarAa. The genes corresponding to components of carbazole 1,9a-dioxygenase from P. stutzeri were cloned and expressed by salt induction in E. coli BL21-SI (a host that allows the enhancement of overexpressed proteins in the soluble fraction), using the vector pDEST™14. The expression of these proteins was performed under different induction conditions (cell concentration, temperature, and time), with the help of two-level factorial design. Cell concentration at induction (measured by absorbance at 600 nm) was tested at 0.5 and 0.8. After salt induction, expression was performed at 30 and 37°C, for 4 h and 24 h. Protein expression was evaluated by densitometry analysis. Expression of CarAa was enhanced by induction at a lower cell concentration and temperature and over a longer time, according to the analysis of the experimental design results. The results were validated at Abs ind = 0.3, 25°C, and 24 h, at which CarAa expression was three times higher than under the standard condition. The behavior of CarAc and CarAd was the inverse, with the best co-expression condition tested being the standard one (Abs ind = 0.5, T = 37°C, and t = 4 h). The functionality of the proteins expressed in E. coli was confirmed by the degradation of 20 ppm carbazole.


Similar content being viewed by others

References
Baneyx F (1999) Recombinant protein expression in Escherichia coli. Curr Opin Biotechnol 10:411–421. doi:10.1016/S0958-1669(99)00003-8
Benedik MJ, Gibbs PR, Riddle RR, Willson RC (1998) Microbial denitrogenation of fossil fuels. Trends Biotechnol 16:390–395. doi:10.1016/S0167-7799(98)01237-2
Bhandari P, Gowrishankar J (1997) An Escherichia coli host strain useful for efficient overproduction of cloned gene products with NaCl as the inducer. J Bacteriol 179:4403–4406
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Cao Y, Xia Q, Fang B (2006) Optimization of expression of dhaT gene encoding 1,3-propanediol oxidoreductase from Klebsiella pneumoniae in Escherichia coli using the methods of uniform design and regression analysis. J Chem Technol Biotechnol 81:109–112. doi:10.1002/jctb.1360
Chen Y, Xing X-H, Ye F, Kuang Y, Luo M (2007) Production of MBP-HepA fusion protein in recombinant Escherichia coli by optimization of culture medium. Biochem Eng J 34:114–121. doi:10.1016/j.bej.2006.11.020
Choi WC, Oh BC, Kim HK, Lee ES, Oh TK (2002) Medium optimization for phytase production by recombinant Escherichia coli using statistical experimental design. J Microbiol Biotechnol 12:490–496
Chuan YP, Lua LHL, Middelberg APJ (2008) High-level expression of soluble viral structural protein in Escherichia coli. J Biotechnol 134:64–71. doi:10.1016/j.jbiotec.2007.12.004
De León A, Jiménez-Islas H, González-Cuevas M, Barba de la Rosa AP (2004) Analysis of the expression of the Trichoderma harzianum ech42 gene in two isogenic clones of Escherichia coli by surface response methodology. Process Biochem 39:2173–2178. doi:10.1016/j.procbio.2003.11.013
Donovan RS, Robinson CW, Glick BR (1996) Optimizing inducer and culture conditions for expression of foreign proteins under the control of the lac promoter. J Ind Microbiol Biotechnol 16:145–154. doi:10.1007/BF01569997
Fontani S, Niccolai A, Kapat A, Olivieri R (2003) Studies on the maximization of recombinant Helicobacter pylori neutrophil-activating protein production in Escherichia coli: application of Taguchi robust design and response surface methodology for process optimization. World J Microbiol Biotechnol 19:711–717. doi:10.1023/A:1025104119260
García-Arrazola R, Dawson P, Buchanan I, Doyle B, Fearn T, Titchener-Hooker N, Baganz F (2005) Evaluation of the effects and interactions of mixing and oxygen transfer on the production of Fab’ antibody fragments in Escherichia coli fermentation with gas blending. Bioprocess Biosyst Eng 27:365–374. doi:10.1007/s00449-005-0414-4
Han GH, Shin H-J, Kim SW (2008) Optimization of bio-indigo production by recombinant E. coli harboring fmo gene. Enzyme Microb Technol 42:617–623. doi:10.1016/j.enzmictec.2008.02.004
Hao DC, Zhu PH, Yang SL, Yang L (2006) Optimization of recombinant Cytochrome P450 2C9 protein production in Escherichia coli DH5α by statistically-based experimental design. World J Microbiol Biotechnol 22:1169–1176. doi:10.1007/s11274-006-9158-9
Hao DC, Zhu PH, Yang SL, Yang L (2007) Enhanced production of human Cytochrome P450 2C9 by Escherichia coli BL21(DE3)pLysS through the novel use of grey relational analysis and Plackett–Burman design. World J Microbiol Biotechnol 23:71–78. doi:10.1007/s11274-006-9194-5
Hernández VEB, Maldonado LMTP, Rivero EM, Barba de la Rosa AP, Acevedo LGO, De León Rodríguez A (2008) Optimization of human interferon gamma production in Escherichia coli by response surface methodology. Biotechnol Bioprocess Eng 13:7–13. doi:10.1007/s12257-007-0126-5
Hisatsuka K, Sato M (1994) Microbial transformation of carbazole to anthranilic acid by Pseudomonas stutzeri. Biosci Biotechnol Biochem 58:213–214. doi:10.1271/bbb.58.213
Hounsa CG, Aubry JM, Dubourguier HC, Hornez JP (1996) Application of factorial and Doehlert designs for optimization of pectate lyase production by a recombinant Escherichia coli. Appl Microbiol Biotechnol 45:764–770. doi:10.1007/s002530050760
Islam RS, Tisi D, Levy MS, Lye GJ (2007) Framework for the rapid optimization of soluble protein expression in Escherichia coli combining microscale experiments and statistical experimental design. Biotechnol Prog 23:785–793. doi:10.1021/bp070059a
Jana S, Deb JK (2005) Strategies for efficient production of heterologous proteins in Escherichia coli. Appl Microbiol Biotechnol 67:289–298. doi:10.1007/s00253-004-1814-0
Kilbane II JJ (2004) Petroleum biorefining: the selective removal of sulfur, nitrogen, and metals. In: Vazquez-Duhalt R, Quintero-Ramirez R (eds) Petroleum biotechnology, vol 151: development and perspectives, studies in surface science and catalysis, chap 2. Elsevier, Mexico City
Kotik M, Kocanová M, Maresová H, Kyslík P (2004) High-level expression of a fungal pyranose oxidase in high cell-density fed-batch cultivations of Escherichia coli using lactose as inducer. Protein Expr Purif 36:61–69. doi:10.1016/j.pep.2004.02.011
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. doi:10.1038/227680a0
Larentis AL, Almeida RV, Cardoso AM, Almeida WI, Rössle SC, Bisch PM, Martins OB, Alves TLM (2006) Homology modeling of the oxygenase component of carbazole 1,9a-dioxygenase (CarAa) involved in petroleum denitrogenation pathway of Pseudomonas sp. Braz Arch Biol Technol 49:53–61
Larentis AL, Almeida RV, Rössle SC, Cardoso AM, Almeida WI, Bisch PM, Alves TLM, Martins OB (2006) Expression and homology modeling of 2′-aminobiphenyl-2,3-diol 1,2-dioxygenase (CarB) from Pseudomonas stutzeri carbazole degradation pathway. Cell Biochem Biophys 44:530–538. doi:10.1385/CBB:44:3:530
Larentis AL, Alves TLM, Martins OB (2005) Cloning and expression of meta-cleavage enzyme (CarB) of carbazole degradation pathway from Pseudomonas stutzeri. Braz Arch Biol Technol 48:127–134. doi:10.1590/S1516-89132005000400016
Lee KM, Rhee CH, Kang CK, Kim JH (2006) Sequential and simultaneous statistical optimization by dynamic design of experiment for peptide overexpression in recombinant Escherichia coli. Appl Biochem Biotechnol 135:59–80. doi:10.1385/ABAB:135:1:59
Lee KM, Rhee CH, Kang CK, Kim JH (2006) Statistical medium formulation and process modeling by mixture design of experiment for peptide overexpression in recombinant Escherichia coli. Appl Biochem Biotechnol 135:81–100. doi:10.1385/ABAB:135:1:81
Leite LF, Neto JNN, Bevilaqua JV (2005) Biorefineries and biofuels: current activities and future vision of Petrobras. ACS Div Fuel Chem 50:726–727
Lo PK, Hassan O, Ahmad A, Muhammad Mahadi N, Illias RM (2007) Excretory over-expression of Bacillus sp. G1 cyclodextrin glucanotransferase (CGTase) in Escherichia coli: optimization of the cultivation conditions by response surface methodology. Enzyme Microb Technol 40:1256–1263. doi:10.1016/j.enzmictec.2006.09.020
Makrides SC (1996) Strategies for achieving high-level expression of genes in Escherichia coli. Microbiol Rev 60:512–538
Maldonado LMTP, Hernández VEB, Rivero EM, Barba de la Rosa AP, Flores JLF, Acevedo LGO, De León Rodríguez A (2007) Optimization of culture conditions for a synthetic gene expression in Escherichia coli using response surface methodology: the case of human interferon beta. Biomol Eng 24:217–222. doi:10.1016/j.bioeng.2006.10.001
Manderson D, Dempster R, Chisti Y (2006) A recombinant vaccine against hydatidosis: production of the antigen in Escherichia coli. J Ind Microbiol Biotechnol 33:173–182. doi:10.1007/s10295-005-0046-3
Niccolai A, Fontani S, Kapat A, Olivieri R (2003) Maximization of recombinant Helicobacter pylori neutrophil activating protein production in Escherichia coli: improvement of a chemically defined medium using response surface methodology. FEMS Microbiol Lett 221:257–262. doi:10.1016/S0378-1097(03)00184-8
Nikerel İE, Toksoy E, Kirdar B, Yildirim R (2005) Optimizing medium composition for TaqI endonuclease production by recombinant Escherichia coli cells using response surface methodology. Process Biochem 40:1633–1639. doi:10.1016/j.procbio.2004.06.017
Oliveira C, Costa S, Teixeira JA, Domingues L (2009) cDNA cloning and functional expression of the α-d-galactose-binding lectin frutalin in Escherichia coli. Mol Biotechnol 43:212–220. doi:10.1007/s12033-009-9191-7
Pan H, Xie Z, Bao W, Zhang J (2008) Optimization of culture conditions to enhance cis-epoxysuccinate hydrolase production in Escherichia coli by response surface methodology. Biochem Eng J 42:133–138. doi:10.1016/j.bej.2008.06.007
Pan HF, Bao WN, Xie ZP, Zhang JG (2010) Optimization of medium composition for cis-epoxysuccinate hydrolase production in Escherichia coli by response surface methodology. Afr J Biotechnol 9:1366–1373
Park H-S, Kayser KJ, Kwak JH, Kilbane JJ II (2004) Heterologous gene expression in Thermus thermophilus: β-galactosidase, dibenzothiophene monooxygenase, PNB carboxy esterase, 2-aminobiphenyl-2,3-diol dioxygenase, and chloramphenicol acetyl transferase. J Ind Microbiol Biotechnol 31:189–197. doi:10.1007/s10295-004-0130-0
Pistorino M, Pfeifer BA (2009) Efficient experimental design and micro-scale medium enhancement of 6-deoxyerythronolide B production through Escherichia coli. Biotechnol Prog 25:1364–1371. doi:10.1002/btpr.250
Ren X, Yu D, Han S, Feng Y (2006) Optimization of recombinant hyperthermophilic esterase production from agricultural waste using response surface methodology. Bioresour Technol 97:2345–2349. doi:10.1016/j.biortech.2005.10.027
Riddle RR, Gibbs PR, Willson RC, Benedik MJ (2003) Recombinant carbazole-degrading strains for enhanced petroleum processing. J Ind Microbiol Biotechnol 30:6–12. doi:10.1007/s10295-002-0005-1
Rodrigues MI, Iemma AF (2005) Planejamento de experimentos e otimização de processos: uma estratégia seqüencial de planejamentos. Casa do Pão Editora, Campinas
Sato SI, Nam J-W, Kasuga K, Nojiri H, Yamane H, Omori T (1997) Identification and characterization of genes encoding carbazole 1,9a-dioxygenase in Pseudomonas sp. strain CA10. J Bacteriol 179:4850–4858
Shin CS, Hong MS, Bae CS, Lee J (1997) Enhanced production of human mini-proinsulin in fed-batch cultures at high cell density of Escherichia coli BL21(DE3)[pET-3aT2M2]. Biotechnol Prog 13:249–257. doi:10.1021/bp970018m
Sørensen HP, Mortensen KK (2005) Advanced genetic strategies for recombinant protein expression in Escherichia coli. J Biotechnol 115:113–128. doi:10.1016/j.jbiotec.2004.08.004
Sunitha K, Kim Y-O, Lee J-K, Oh T-K (2000) Statistical optimization of seed and induction conditions to enhance phytase production by recombinant Escherichia coli. Biochem Eng J 5:51–56. doi:10.1016/S1369-703X(99)00062-5
Sunitha K, Lee J-K, Oh T-K (1999) Optimization of medium components for phytase production by E. coli using response surface methodology. Bioprocess Biosyst Eng 21:477–481. doi:10.1007/PL00009086
Swalley SE, Fulghum JR, Chambers SP (2006) Screening factors effecting a response in soluble protein expression: formalized approach using design of experiments. Anal Biochem 351:122–127. doi:10.1016/j.ab.2005.11.046
Terpe K (2006) Overview of bacterial expression systems for heterologous protein production: from molecular and biochemical fundamentals to commercial systems. Appl Microbiol Biotechnol 72:211–222. doi:10.1007/s00253-006-0465-8
Urban A, Ansmant I, Motorin Y (2003) Optimisation of expression and purification of the recombinant Yol066 (Rib2) protein from Saccharomyces cerevisiae. J Chromatogr B 786:187–195. doi:10.1016/S1570-0232(02)00742-0
Volontè F, Marinelli F, Gastaldo L, Sacchi S, Pilone MS, Pollegioni L, Molla G (2008) Optimization of glutaryl-7-aminocephalosporanic acid acylase expression in E. coli. Protein Expr Purif 61:131–137. doi:10.1016/j.pep.2008.05.010
Wang Y-h, Jing C-f, Yang B, Mainda G, Dong M-l, Xu A-l (2005) Production of a new sea anemone neurotoxin by recombinant Escherichia coli: optimization of culture conditions using response surface methodology. Process Biochem 40:2721–2728. doi:10.1016/j.procbio.2004.12.024
Zhang X, Li Y, Zhuge B, Tang X, Shen W, Rao Z, Fang H, Zhuge J (2006) Optimization of 1,3-propanediol production by novel recombinant Escherichia coli using response surface methodology. J Chem Technol Biotechnol 81:1075–1078. doi:10.1002/jctb.1538
Zhao J, Wang Y, Chu J, Zhang S, Zhuang Y, Yuan Z (2008) Statistical optimization of medium for the production of pyruvate oxidase by the recombinant Escherichia coli. J Ind Microbiol Biotechnol 35:257–262. doi:10.1007/s10295-007-0301-x
Acknowledgments
CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and Petrobras supported this work. We thank to Iuri Bastos M.Sc. (UFRJ) for the densitometry analysis using the QuantiScan 1.25 program, Dr. Márcio Schwaab and Prof. José Carlos Pinto (COPPE/UFRJ) for fruitful discussions, and the reserchers of the Setor de Imunobiológicos laboratory at INCQS/Fiocruz for making available the QuantiOne 4.4.1 program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Larentis, A.L., Sampaio, H.C.C., Martins, O.B. et al. Influence of induction conditions on the expression of carbazole dioxygenase components (CarAa, CarAc, and CarAd) from Pseudomonas stutzeri in recombinant Escherichia coli using experimental design. J Ind Microbiol Biotechnol 38, 1045–1054 (2011). https://doi.org/10.1007/s10295-010-0879-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-010-0879-2