Abstract
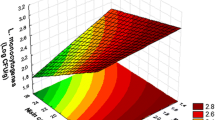
Food producers apply modern processing techniques and use a variety of preservative additives to guarantee safe food and a longer shelflife. Regrettably many of these impact the sensory characteristics of the foodstuffs, such as colour, texture, and flavour, which can result in low consumer acceptance. Additionally, strategies used to reduce growth of spoilage and pathogenic bacteria are not selective enough and may inactivate also desired microbiota. Food is usually overdosed with antimicrobials that are supplemented ‘just in case.’ Consequently, food producers are searching for natural preservation methods that are not harmful to humans. Nature offers a wide spectrum of biologically active (phyto) chemicals that can be used as potential natural preservatives. Compounds with bacterial growth-limiting properties are detected in all parts of plants, including their leaves, flowers, fruits, roots, etc. These are mostly acids, alcohols, medium and long-chain organic acids, terpenic compounds, and their derivatives. This study focused on the effectiveness of plant extracts, i.e., synergism between terpenoids and medium chain fatty acids in cured cooked meat. Bacterial strains that were tested include typical members of the spoilage microflora in vacuum (Lactobacillus curvatus) and MA-packed meats (Brochothrix thermosphacta). These were isolated and identified in a separate study. L. curvatus was observed to be very resistant against either terpenoids or fatty acids when used separately, whereas its growth was strongly inhibited when both chemicals were combined. Growth of B. thermosphacta was significantly inhibited when antimicrobial compounds were solely applied, whereas a blend of terpenoids and fatty acids showed an almost bactericidal effect.
Similar content being viewed by others
Notes
Chemicals (terpenoid compounds and fatty acids) were purchased in Aldrich, Belgium.
References
Marino M, Bersani C, Comi G (2001) Impedance measurements to study the antimicrobial activity of essential oils from Lamiaceae and Compositae. Int J Food Microbiol 67:187–195
Aligannis N, Kalpoutzakis E, Mitaku S, Chinou B (2001) Composition and antimicrobial activity of the essential oils of two Origanum species. J Agric Food Chem 49:4168–4170
Burt S (2004) Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol 94:223–253
Nasar-Abbas SM, Kadir Halkman A (2004) Antimicrobial effect of water extract of sumac (Rhus coriaria L.) on the growth of some food borne bacteria including pathogens. Int J Food Microbiol 97:63–69
Sagdıc O, Ozcan M (2003) Antibacterial activity of Turkish spice hydrosols. Food Control 14:141–143
Lambert RJW, Skandamis PN, Coote PJ, Nychas GJE (2001) A study of minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J Appl Microbiol 91:453–462
Bagamboula CF, Uyttendaele M, Debevere J (2004) Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S. Flexneri. Food Microbiol 21:33–42
Yamazaki K, Yamamoto T, Kawai Y, Inoue N (2004) Enhancement of antilisterial activity of essential oil constituents. Food Microbiol 21:283–289
Delaquois PJ, Stanich K, Girard B, Mazza G (2002) Antimicrobial activity of individual and mixed fractions of dill, cilantro, coriander and eucalyptus essential oils. Int J Food Microbiol 74:101–109
Ultee A, Kets EPW, Alberda M (2000) Adaptation of the food-borne pathogen Bacillus cereus to carvacrol. Arch Microbiol 174:233–238
Fernandez-Lopez J, Zhi N, Aleson-Carbonel L, Perez-Alvarez JA, Kuri V (2005) Antioxidant and antibacterial activities of natural extracts: application in beef meatballs. Meat Sci 69:371–380
Lin YT, Labbe RG, Shetty K (2004) Inhibition of Listeria monocytogenes in fish and meat systems by use of oregano and cranberry phytochemical synergies. Appl Environ Microbiol 70:5672–5678
Lin J, Smith MP, Chapin KC, Baik HS, Bennett GN, Foster JW (1996) Mechanisms of acid resistance in enterohemorrhagic Escherichia coli. Appl Environ Microbiol 62:3094–3100
Ultee A, Bennik MHJ, Moezelaar R (2002) The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl Environ Microbiol 68:1561–1568
Nascimento GGF, Locatelli J, Freitas PC, Silva GL (2000) Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Braz J Microbiol 31:247–256
Huhtanen CN (1980) Inhibition of Clostridium botulinum by spice extracts and aliphatic alcohols. J Food Prot 43:195–196
Nakai SA, Siebert KJ (2004) Organic acid inhibition models for Listeria innocua, Listeria ivanovii, Pseudomonas aeruginosa and Oenococcus oeni. Food Microbiol 21:67–72
Immerseel F, De Buck J, Boyen F, Bohez L, Pasmans F, Volf J, Sevcik M, Rychlik I, Haesebrouck F, Ducatelle R (2004) Medium-chain fatty acids decrease colonization and invasion through hilA suppression shortly after infection of chickens with Salmonella enterica serovar enteritidis. Appl Environ Microbiol 70:3582–3587
Davies EA, Milne CF, Bevis HE, Potter RW, Harris JM, Williams GC, Thomas LV, Delves-Broughton J (1999) Effective use of nisin to control lactic acid bacterial spoilage in vacuum-packed Bologna-type sausage. J Food Prot 62:1004–1010
Greenacre EJ, Brocklehurst TF, Waspe CR, Wilson DR, Wilson PD (2003) G. Salmonella enterica Serovar Typhimurium and Listeria monocytogenes acid tolerance response induced by organic acids at 20°C: optimization and modelling. Appl Environ Microbiol 69:3945–3951
Sakamoto K, Veen HW, Saito H, Kobayashi H, Konings WN (2002) Membrane bound ATPase contributes to hop resistance of Lactobacillus brevis. Appl Environ Microbiol 68:5374–5378
Jacobson JM, Feinman L, Liebes L, Ostrow N, Koslowski V, Tobia A, Cabana BE, Lee D (2001) Pharmacokinetics, safety, and antiviral effects of hypericin, a derivative of St. John’s wort plant in patients with chronic hepatitis C virus infection. Antimicrob Agents Chemother 45:517–524
Kuo YH, Li SY, Huang RL, Wu MD, Huang HC, Lee KH (2001) Schizarin B, C, D, and E, four new lignans from Kadsura matsudai and their antihepatitis activities. J Nat Prod 64:487–490
Ng TB, Wang H (2001) Panaxagin, a new protein from Chinese ginseng possesses anti-fungal, antiviral, translation-inhibiting and ribonuclease activities. Life Sci 68:739–749
Shirataki Y, Motohashi N, Tani S, Sakagami H, Satoh K, Nakashima H, Mahapatra SK, Ganguly K (2001) In vitro biological activity of prenylflavanones. Anticancer Res 21:275–280
Mbandi E, Brywig M, Shelef LA (2004) Antilisterial effects of free fatty acids and monolaurin in beef emulsions and hot dogs. Food Microbiol 21:815–818
Dorman HJD, Deans SG (2000) Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol 88:308–316
Marounek M, Skrivanova E, Rada V (2003) Susceptibility of Escherichia coli to C2–C18 fatty acids. Folia Microbiol 48:731–735
Ultee A, Kets EPW, Smid EJ (1999) Mechanism of action of carvacrol on the food-borne Bacillus cereus. Appl Environ Microbiol 65:4606–4610
Helander IM, Alakomi HL, Latva-Kala K, Mattila-Sandholm T, Pol I, Smid EJ, Gorris LGM, von Wright A (1998) Characterization of the action of selected essential oil components on Gram-Negative bacteria. J Agric Food Chem 46:3590–3595
Chattopadhyay MK, Jagannadham MV (2003) A branched chain fatty acid promotes cold adaptation in bacteria. J Biosci 28:363–364
Annous BA, Kozempel MF, Kurantz MJ (1999) Changes in membrane fatty acid composition of pediococcus sp. strain nrrl b-2354 in response to growth conditions and its effect on thermal resistance. Appl Environ Microbiol 65:2857–2862
Annous BA, Becker LA, Bayles DO, Labeda DP, Wilkinson BJ (1997) Critical role of anteiso-c15:0 fatty acid in the growth of Listeria monocytogenes at low temperatures. Appl Environ Microbiol 63:3887–3894
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the BioMicroWorld 2009 Special Issue.
Rights and permissions
About this article
Cite this article
Szczepaniak, S., Polanska, M., Van Assche, A. et al. The synergism of natural compounds in the pursuit of safe and healthier food. J Ind Microbiol Biotechnol 38, 215–220 (2011). https://doi.org/10.1007/s10295-010-0822-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-010-0822-6