Abstract
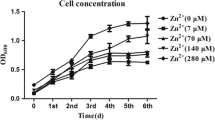
The effect of trace metal ions (Co2+, Cu2+, Fe2+, Mn2+, Mo6+, Ni2+, Zn2+, SeO4 − and WO4 −) on growth and ethanol production by an ethanologenic acetogen, Clostridium ragsdalei was investigated in CO:CO2-grown cells. A standard acetogen medium (ATCC medium no. 1754) was manipulated by varying the concentrations of trace metals in the media. Increasing the individual concentrations of Ni2+, Zn2+, SeO4 − and WO4 − from 0.84, 6.96, 1.06, and 0.68 μM in the standard trace metals solution to 8.4, 34.8, 5.3, and 6.8 μM, respectively, increased ethanol production from 35.73 mM under standard metals concentration to 176.5, 187.8, 54.4, and 72.3 mM, respectively. Nickel was necessary for growth of C. ragsdalei. Growth rate (μ) of C. ragsdalei improved from 0.34 to 0.49 (day−1), and carbon monoxide dehydrogenase (CODH) and hydrogenase (H2ase)-specific activities improved from 38.45 and 0.35 to 48.5 and 1.66 U/mg protein, respectively, at optimum concentration of Ni2+. At optimum concentrations of WO4 − and SeO4 −, formate dehydrogenase (FDH) activity improved from 32.3 to 42.6 and 45.4 U/mg protein, respectively. Ethanol production and the activity of FDH reduced from 35 mM and 32.3 U/mg protein to 1.14 mM and 8.79 U/mg protein, respectively, upon elimination of WO4 − from the medium. Although increased concentration of Zn2+ enhanced growth and ethanol production, the activities of CODH, FDH, H2ase and alcohol dehydrogenase (ADH) were not affected by varying the Zn2+ concentration. Omitting Fe2+ from the medium decreased ethanol production from 35.7 to 6.30 mM and decreased activities of CODH, FDH, H2ase and ADH from 38.5, 32.3, 0.35, and 0.68 U/mg protein to 9.07, 7.01, 0.10, and 0.24 U/mg protein, respectively. Ethanol production improved from 35 to 54 mM when Cu2+ was removed from the medium. The optimization of trace metals concentration in the fermentation medium improved enzyme activities (CODH, FDH, and H2ase), growth and ethanol production by C. ragsdalei.



Similar content being viewed by others
References
Adams MWW, Mortenson LE, Chen J-S (1981) Hydrogenase. Biochem Biophys Acta 594:105–176
Balch WE, Wolfe RS (1976) New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressurized atmosphere. Appl Environ Microbiol 32:781–791
Bramlett MR, Tan X, Lindahl PA (2003) Inactivation of acetyl CoA synthase/carbon monoxide dehydrogenase by copper. J Am Chem Soc 125:9316–9317
Burdette DS, Jung S-H, Shen G-J, Hollingsworth RI, Zeikus JG (2002) Physiological function of alcohol dehydrogenase and long-chain (C30) fatty acids in alcohol tolerance of Thermoanaerobacter ethanolicus. Appl Environ Microbiol 68:1914–1918
Cammack R (1999) Hydrogenase sophistication. Nature 397:214–215
Chen JS (1995) Alcohol dehydrogenase: multiplicity and relatedness in the solvent-producing clostridia. FEMS Microbiol Rev 17:263–273
Clark JE, Ragsdale SW, Ljungdahl LG, Wiegel J (1982) Levels of enzymes involved in the synthesis of acetate from carbon dioxide in Clostridium thermoautotrophicum. J Bacteriol 151:507–509
Diekert GB, Thauer RK (1980) The effect of nickel on carbon monoxide dehydrogenase formation in Clostridium thermoaceticum and Clostridium formicoaceticum. FEMS Microbiol Lett 7:187–189
Drake HL, Kusel K, Matthies C (2006) Acetogenic prokaryotes. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (eds) The prokaryotes, 3rd edn. Springer, Berlin Heidelberg New York, pp 354–420
Drennan CL, Doukov TI, Ragsdale SW (2004) The metalloclusters of carbon monoxidedehydrogenase/acetyl-CoA synthase: a story in pictures. J Biol Inorg Chem 9:511–515
Hill J, Nelson E, Tilman D, Polasky S, Tiffany D (2006) Environmental, economic, and energetic costs and benefits of biodiesel and ethanol biofuels. Proc Natl Acad Sci 103:11206–11210
Huhnke R, Lewis R, Tanner RS (2008) Isolation and characterization of novel clostridial species. US patent application. Publication no. US 2008/0057554 A1
Ismaiel AA, Zhu CX, Colby GD, Chen JS (1993) Purification and characterization of a primary-secondary alcohol dehydrogenase from two strains of Clostridium beijerinckii. J Bacteriol 175:5097–5105
Koesnandar NN, Nagai S (1991) Effects of trace metal ions on the growth, homoacetogenesis and corronoid production by Clostridium aceticum. J Fermen Bioengg 71:181–185
Korkhin Y, Kalb (Gilboa) AJ, Peretz M, Bogin O, Burstein Y, Frolow F (1998) NADP-dependent bacterial alcohol dehydrogenases: crystal structure, cofactor-binding and cofactor specificity of the ADHs of Clostridium beijerinckii and Thermoanaerobacter brockii. J Mol Biol 278:967–981
Liu C-L, Mortenson LE (1984) Formate dehydrogenase of Clostridium pasteurianum. J Bacteriol 159:375–380
Margeot A, Hahn-Hagerdal B, Edlund M, Slade R, Monot F (2009) New improvements for lignocellulosic ethanol. Curr Opin Biotechnol 20:372–380
Mehta MD, Saxena J, Tanner RS (2004) Enzyme activities in clostridia producing ethanol from carbon monoxide. Abstr 104th Annu Meet Am Soc Microbiol, O-81, p 474
Mielenz JR (2001) Ethanol production from biomass: technology and commercialization status. Curr Opin Microbiol 4:324–329
Peckman KR (1976) Investigation of the phylogenetic relationship of Sporomusa ureae to members of the Bacillaceae using primary structural characterization of 16S ribosomal ribonucleic acids. PhD thesis. University of Illinois, Urbana
Perlack RD, Wright LL, Turhollow AF, Graham RL, Stokes BJ, Erbach DC (2005) Biomass as feedstock for a bioenergy and bioproducts industry: the technical feasibility of a billion-ton annual supply. DOE/GO-102005-2135, Oak Ridge National Laboratory, Oak Ridge. http://:www.osti.gov/bridge
Peters JW, Lanzilotta WN, Lemon BJ, Seefeldt LC (1998) X-ray crystal structure of the Fe-only hydrogenase (CpI) from Clostridium pasteurianum to 1.8 angstrom resolution. Science 5395:1853–1858
Pierce E, Xie G, Barabote RD, Saunders E, Han CS, Detter JC, Richardson P, Brettin TS, Das A, Ljungdahl LG, Ragsdale SW (2008) The complete genome sequence of Moorella thermoacetica (f. Clostridium thermoaceticum). Appl Environ Microbiol 10:2550–2573
Pimentel D (2003) Ethanol fuels: energy balance, economics, and environment impacts are negative. Nat Resour Res 12:127–134
Ragsdale SW, Ljungdahl LG (1984) Hydrogenase from Acetobacterium woodii. Arch Microbiol 139:361–365
Scopes RK (1983) An iron-activated alcohol dehydrogenase. FEBS Lett 156:303–306
Seravalli J, Xiao Y, Gu W, Cramer SP, Antholine WE, Krymov V, Gerfen GJ, Ragsdale SW (2004) Evidence that NiNi acetyl-CoA synthase is active and that the CuNi enzyme is not. Biochem 43:3944–3955
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Garter FH, Provenzano MD, Fujimoto EK, Goeke MN, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Tanner RS (2007) Cultivation of bacteria and fungi. In: Hurst CJ, Crawford RL, Mills AL, Garland JL, Stetzenbach LD, Lipson DA (eds) Manual of environmental microbiology, 3rd edn. ASM Press, Washington, DC, pp 69–78
Tanner RS (2008) Production of ethanol from synthesis gas. In: Wall J, Harwood CJ, Demain AL (eds) Bioenergy. ASM Press, Washington, DC, pp 147–151
Wagner R, Andreesen JR (1987) Accumulation and incorporation of 185W-tungsten into proteins of Clostridium acidiurici and Clostridium cylindrosporum. Arch Microbiol 147:295–299
Yamamoto I, Saiki T, Liu SM, Ljungdahl LG (1983) Purification and properties of NADP-dependent formate dehydrogenase from Clostridium thermoaceticum, a tungsten-selenium-iron protein. J Biol Chem 258:1826–1832
Acknowledgments
This research was supported in part by USDA-CSREES Special Grant awards 2005-34447-15711 and 2006-34447-16939. We would like to thank Dr. James A. Zahn for his constructive suggestions in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saxena, J., Tanner, R.S. Effect of trace metals on ethanol production from synthesis gas by the ethanologenic acetogen, Clostridium ragsdalei . J Ind Microbiol Biotechnol 38, 513–521 (2011). https://doi.org/10.1007/s10295-010-0794-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-010-0794-6