Abstract
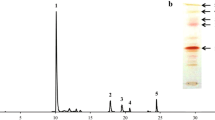
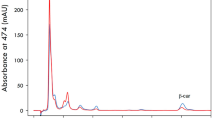
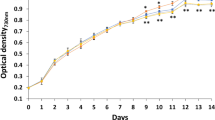
A moderate-temperature mutant strain of the yeast Phaffia rhodozyma, termed MK19, was selected by 1-methyl-3-nitro-1-nitrosoguanidine (NTG) and Co60 mutagenesis. MK19 displayed fast cell growth and elevated astaxanthin content at 25°C, whereas optimal temperature for growth and astaxanthin synthesis of wild-type P. rhodozyma was 17–21°C. Optimized astaxanthin yield for MK19 after 4 days culture in shaking flask at 25°C, determined by response surface methodology, was 25.8 mg/l, which was 17-fold higher than that of the wild-type. MK19 was tolerant of high initial concentration of glucose (>100 g/l) in optimized medium. Total fatty acid content of MK19 was much lower than that of the wild-type. Acetyl-CoA is a common precursor of fatty acid and terpenoid biosynthesis, and it is possible that decreased fatty acid synthesis results in transfer of acetyl-CoA to the carotenoid biosynthetic pathway. Our results indicate that astaxanthin content is negatively correlated with fatty acid content in P. rhodozyma. Nutrient analysis showed that MK19 cells are enriched in lysine, vitamin E, and other rare nutrients, and have potential application as fish food without nutritional supplementation. This moderate-temperature mutant strain is a promising candidate for economical industrial-scale production.




Similar content being viewed by others
References
Andrewes AG, Phaff HJ, Starr MP (1976) Carotenoids of Phaffia rhodozyma, a red-pigmented fermenting yeast. Phytochemistry 15:1003–1007
Calo P, Velazquez JB, Sieiro C, Blanco P, Longo E, Villa TG (1995) Analysis of astaxanthin and other carotenoids from several Phaffia rhodozyma mutants. J Agric Food Chem 43:1396–1399
Diego L, Martín M, Virginia de G, Sonia F, María van B (2008) Characterization of a novel South American population of the astaxanthin producing yeast Xanthophyllomyces dendrorhous (Phaffia rhodozyma). J Ind Microbiol Biotechnol 35:151–158
Gerry PQ, MiChael JK (2002) Experimental design and data analysis for biologists. Printed in the United Kingdom at the University Press, Cambridge
Ginka IF, Dora MB (2009) Carotenoids from Rhodotorula and Phaffia: yeasts of biotechnological importance. J Ind Microbiol Biotechnol 36:163–180
Haard NF (1988) Astaxanthin formation by the yeast Phaffia rhodozyma on molasses. Biotechnol Lett 10:609–614
Higuera-Ciapara I, Félix-Valenzuela L, Goycoolea FM (2006) Astaxanthin: a review of its chemistry and applications. Crit Rev Food Sci Nutr 46:185–196
Hu ZC, Zheng YG, Wang Z, Shen YC (2005) Effect of sugar-feeding strategies on astaxanthin production by Xanthophyllomyces dendrorhous. World J Microbiol Biotechnol 21:771–775
Hu ZC, Zheng YG, Wang Z, Shen YC (2006) pH control strategy in astaxanthin fermentation bioprocess by Xanthophyllomyces dendrorhous. Enzyme Microb Technol 39:586–590
Hu ZC, Zheng YG, Wang Z, Shen YC (2007) Production of Astaxanthin by Xanthophyllomyces dendrorhous ZJUT46 with fed-batch fermentation in 2.0 M3 Fermentor. Food Technol Biotechnol 45(2):209–212
Johnson EA (2003) Phaffia rhodozyma: colorful odyssey. Int Microbiol 6:169–174
Johnson EA, Lewis MJ (1979) Astaxanthin formation by the yeast Phaffia rhodozyma. J Gen Microbiol 115:173–183
Kim J-H, Kang S-W, Kim S-W, Chang H-I (2005) High-level production of astaxanthin by Xanthophyllomyces dendrorhous mutant JH1 using statistical experimental designs. Biosci Biotechnol Biochem 69:1743–1748
Kusdiyantini E, Gaudin P, Goma G, Blanc PJ (1998) Growth kinetics and astaxanthin production of Phaffia rhodozyma on glycerol as a carbon source during batch fermentation. Biotechnol Lett 20:929–934
Mendoza H, Martel A, Jiménez del Río M, Reina García G (1999) Oleic acid is the main fatty acid related with carotenogenesis in Dunaliella salina. J Appl Phycol 11:15–19
Martin G, Mark EH, Miguel O (2003) Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol 21:210–216
Ni H, Chen QH, Ruan H, Yang YF, Li LJ, Wu GB, Hu Y, He GQ (2007) Studies on optimization of nitrogen sources for astaxanthin production by Phaffia rhodozyma. Zhejiang Univ Sci B 8:365–370
Rabbani S, Beyer P, Lintig J, Hugueney P, Kleinig H (1998) Induced beta-carotene synthesis driven by triacylglycerol deposition in the unicellular alga Dunaliella bardawil. Plant Physiol 116:1239–1248
Ramirez J, Gutierrez H, Gschaedler A (2001) Optimization of astaxanthin production by Phaffia rhodozyma through factorial design and response surface methodology. J Biotechnol 88:259–268
Reynders MB, Rawlings DE, Lharrison ST (1996) Studies on the growth, modelling and pigment production by the yeast Phaffia rhodozyma during fed-batch cultivation. Biotechnol Lett 18:649–654
Reynders MB, Rawlings DE, Harrison STL (1997) Demonstration of the crabtree effect in Phaffia rhodozyma during continuous and fed-batch cultivation. Biotechnol Lett 19:549–552
Sengupta S, Modak JM (2001) Optimization of fed-batch bioreactor for immobilized enzyme process. Chem Eng Sci 56:3315–3325
Sun N, Lee S, Song KB (2004) Characterization of a carotenoid-hyperproducing yeast mutant isolated by low-dose gamma irradiation. Int J Food Microbiol 94:263–267
Sukhija PS, Palmquist DL (1988) Rapid method for determination of total fatty acid content and composition of feedstuffs and feces. J Agric Food Chem 36(6):1202–1206
Takashi Y, Tsunehiro A, Yuhsuke M, Takeki Y, Masami S, Seiji K, Kazuhisa O (2007) Nutritional enrichment of larval fish feed with Thraustochytrid producing polyunsaturated fatty acids and xanthophylls. J Biosci Bioeng 104:200–206
Tatsuzawaa H, Maruyamaa T, Misawab N, Fujimoric K, Nakanod M (2000) Quenching of singlet oxygen by carotenoids produced in Escherichia coli—attenuation of singlet oxygen-mediated bacterial killing by Carotenoids. FEBS Lett 484:280–284
Yamane Y, Higashida K, Nakashimada Y, Kakizono T, Nishio N (1997) Astaxanthin production by Phaffia rhodozyma enhanced in fed-batch culture with glucose and ethanol feeding. Biotechnol Lett 19:1109–1111
Yamane Y, Higashida K, Nakashimada Y, Kakizono T, Nishio N (1997) Influence of oxygen and glucose on primary metabolism and astaxanthin production by Phaffia rhodozyma in batch and fed-batch cultures: kinetic and stoichiometric analysis. Appl Environ Microbial 63:4471–4478
Acknowledgments
This study was supported in part by State Training Base for Fundamental Research and Teaching of Biological Sciences program, College of Biological Sciences, China Agricultural University (Grant No. J0730639), and by Beijing Natural Science Foundation Program and Scientific Research Key Program of Beijing Municipal Commission of Education (Grant No. SZ200511417020).
Author information
Authors and Affiliations
Corresponding author
Additional information
L. Miao and Y. Wang contributed equally to this study.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Miao, L., Wang, Y., Chi, S. et al. Reduction of fatty acid flux results in enhancement of astaxanthin synthesis in a mutant strain of Phaffia rhodozyma . J Ind Microbiol Biotechnol 37, 595–602 (2010). https://doi.org/10.1007/s10295-010-0706-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-010-0706-9