Abstract
Combination of physical and chemical mutagenesis was used to isolate hyper secretory strains of Aspergillus niger NCIM 563 for phytase production. Phytase activity of mutant N-1 and N-79 was about 17 and 47% higher than the parent strain. In shake flask the productivity of phytase in parent, mutant N-1 and N-79 was 6,181, 7,619 and 9,523 IU/L per day, respectively. Up scaling of the fermentation from shake flask to 3 and 14 L New Brunswick fermenter was studied. After optimizing various fermentation parameters like aeration, agitation and carbon source in fermentation medium the fermentation time to achieve highest phytase activity was reduced considerably from 14 days in shake flask to 8 days in 14 L fermenter. Highest phytase activity of 80 IU/ml was obtained in 1% rice bran–3.5% glucose containing medium with aeration 0.2 vvm and agitation 550 rpm at room temperature on 8th day of fermentation. Addition of either bavistin (0.1%), penicillin (0.1%), formalin (0.2%) and sodium chloride (10%) in fermented broth were effective in retaining 100% phytase activity for 8 days at room temperature while these reagents along with methanol (50%) and ethanol (50%) confer 100% stability of phytase activity at 4°C till 20 days. Among various carriers used for application of phytase in feed, wheat bran and rice bran were superior to silica and calcium carbonate. Thermo stabilization studies indicate 100% protection of phytase activity in presence of 12% skim milk at 70°C, which will be useful for its spray drying.
Similar content being viewed by others
Introduction
Phytic acid (myo-inositol hexakisphosphate) is the major storage form of phosphorus in cereals and leguminous plants [21], a form that is not readily assimilated by animals. Monogastric animals, especially swine and poultry, lack sufficient amount of intrinsic phytases to hydrolyze the phytic acid complexes in feed [3] which results in release of undigested phytate phosphorus in feces and urine resulting in severe pollution of water resources [19]. Moreover phytic acid has antinutritive properties as it complexes with proteins and nutritionally important metals such as calcium, zinc, magnesium and iron, decreasing their bioavailability [20]. Phytic acid is also known to inhibit a number of nutritionally important enzymes in vivo [9]. Phytases (myo-inositol hexakisphosphate phosphohydrolase; EC 3.1.3.8 and 3.1.3.26) belongs to a subclass of histidine acid phosphatases [18] and catalyzes the hydrolytic degradation of myo-inositol hexakisphosphate (Ins P6) to free inorganic phosphate (Pi), to yield lower myo-inositol phosphate esters (Ins P5–P1) and, in some cases, free myo-inositol, making phosphorus available for bioabsorption [12]. Addition of phytase to poultry feed increases the bioavailability of phosphorus from feed and also reduces the amount of phosphorus in manure [27].
Phytase is present in plants, animal tissues and also produced by large number of bacteria, yeasts and fungi [4, 24, 26]. Among fungi strains of Aspergillus niger (syn. A. ficuum) produce large amounts of extracellular phytase [5] and show more acid tolerance than bacteria and yeasts [13]. In view of its industrial importance the ultimate objective is to produce this enzyme at cost effective level by hyper secretory strains along with effective down streaming and formulation for its industrial application. In the present communication, we report enhanced phytase production by mutant strain N-1 and N-79 as compared to parent strain. The process has also been up scaled from shake flask to 3 and subsequent 14 L New Brunswick fermenter. Stabilization of phytase at room temperature and higher temperature was studied for its application in mash feed and pelleted feed. Earlier, we have reported phytase production under solid-state fermentation (SSF) [16] and submerged fermentation using dextrin–glucose medium [22, 23] and various agriculture residues [2].
Materials and methods
Chemicals
Phytic acid sodium salt was purchased from Sigma Chemical Company, St Louis, MO, USA. All other chemicals used were of analytical grade and obtained from leading manufacturers including Sigma, BDH and Glaxo. Agriculture residues like wheat bran and rice bran were purchased from local market.
Fungal strain
The strain A. niger NCIM 563 used throughout the present study was from NCIM Resource Center, Pune, India. It was maintained on Potato Dextrose Agar (PDA) slants and stored at 4°C.
Isolation of mutant
The spores from 7 days old slant of A. niger NCIM 563 grown on PDA were collected by scraping in 10 ml sterile saline containing 0.01% Tween 80. After addition 0.2% (v/v) ethyl methyl sulfonate (EMS), the suspension was incubated at room temperature for 24 h followed by UV-irradiation, which resulted in 99% killing of spores after exposure for 4 min. Mutants were selected by spreading UV mutated spores on slightly modified phytase screening medium (PSM) agar plates [11] containing 0.5% calcium phytate and 0.05% NaNO3 and selection of colony with enhanced zone of hydrolysis by phytase.
Medium and culture conditions
Fermentation medium for phytase production was according to Bhavsar et al. [2]. Thus modified fermentation medium contained (per 100 ml): rice bran 1 g; glucose 5 g; NaNO3 0.86 g; KCl 0.05 g; MgSO4·7H2O 0.05 g; FeSO4·7H2O 0.01 g, pH 5.5 before sterilization.
Fermentation medium (100 in 250 ml Erlenmeyer flask) was inoculated with 1% (v/v) of spore suspension (5 × 107 spores/ml) prepared by suspending the spores from 7 day old sporulated slant of A. niger NCIM 563 grown on PDA in 10 ml of sterile saline containing 0.01% (v/v) Tween 80 and incubated at 30°C at 200 rpm. Samples were removed after every 24 h and checked for pH, growth, total residual reducing sugar, extra cellular protein and phytase activity.
Fermenter studies
After optimizing the production of phytase under shake flask conditions it was further optimized in 3 and 14 L (New Brunswick, USA) fermenter with working volume of 1.8 and 10 L, respectively. The medium and production conditions were selected on the basis of studies carried out in shake flask level. Thus, medium containing 1% rice bran–5% glucose supplemented with inorganic salts was used. The fermenter was run with aeration of 0.2 vvm and agitation (300–500 rpm), respectively (Fig. 3). Inoculum was prepared by inoculating three 250 ml flasks containing 100 ml of sterile agriculture residue containing medium with 5 × 107 spores/ml and incubating at 30°C at 200 rpm for 72 h. The fermenter containing 1.8 L medium was in situ sterilized and then inoculated aseptically. The fermenter was equipped with different controls such as pH, temperature, dissolved oxygen, agitation and antifoam. Samples were withdrawn at regular time intervals and analyzed for protein content, residual sugar and phytase activity. The process was further up scaled to 14 L fermenter using 1% rice bran and 4% glucose supplemented with salts with aeration and agitation at 0.2 vvm and 400 rpm, respectively.
Phytase assay
Phytase activity was measured at 50°C as described earlier [16]. The reaction was carried out at pH 2.5 using 100 mM glycine–HCl buffer at 50°C for 30 min. The liberated inorganic phosphate was measured by a modification of the ammonium molybdate method [10]. A freshly prepared 4 ml solution of acetone:5 N H2SO4:10 mM ammonium molybdate (2:1:1 v/v/v) and 400 μl of 1 M citric acid were added to the assay mixture. Absorbance was measured at 370 nm. One unit of phytase activity (IU) was expressed as the amount of enzyme that liberates 1 μmol phosphorus/min under standard assay conditions.
Each experiment was carried out in triplicate and the values reported are the mean of three such experiments in which a maximum of 3–5% variability was observed.
Protein estimation
Protein concentration in the culture filtrate was determined by the method of Lowry et al. [15] using Bovine serum albumin as a standard.
Sugar content
Total residual reducing sugar concentration was estimated by DNSA method [17] and HPLC system (Dionex India Limited, Mumbai, India) equipped with UV- or RI-detectors. An ion exclusion column (Aminex HPX-87H; Bio-Rad, Hercules, CA, USA) was used at a temperature of 38°C with 8 mM H2SO4 as a mobile phase at a flow rate of 6 ml/min.
Stability of phytase in presence of various additives
To determine the stability of phytase in presence of various additives the fermented broth was supplemented with various additives like solvents, antibiotics, formalin, glycine and sodium chloride for different time intervals at room temperature and 8–10°C and residual activity was evaluated.
Release of inorganic phosphorus from poultry feed ingredients
To study effect of phytase in removal of phytates from poultry feed ingredients 1 g of each agriculture residue was suspended in 10 ml glycine–HCl buffer (100 mM pH 2.5). To this 1 ml of phytase enzyme (272 IU) is added followed by incubation at 39°C for 2 h to simulate the condition of poultry stomach. The samples were removed after various time intervals up to 2 h and checked for the release of inorganic phosphate from agriculture residue by action of enzyme using ammonium molybdate method [10]. At the same time initial phosphorus of agriculture residue was also checked.
Formulation of phytase in powder form
To evaluate the stability of phytase in powder form the concentrated enzyme was mixed with various carriers, dried to obtain free flowing material and activity was monitored. Thus 4 ml concentrated enzyme (232 IU) was mixed with 10 g sterilized carrier like wheat bran, rice bran, calcium carbonate and silica and dried at 50°C for 2 h (moisture content <4%). Samples were removed after various time intervals and evaluated for phytase activity.
Results and discussions
Isolation of mutants
More than 1,200 mutants were isolated on plates containing 0.5% calcium phytate. Mutants were selected on the basis of small compact colony with large zone of hydrolysis on calcium phytate plate as compared to parent strain. All the positive mutants were quantified for phytase production using rice bran–glucose–salt medium in shake flask condition. Mutants N-1 and N-79 were found to be superior to parent strain as they produce 80 and 100 IU/ml phytase activities on 10.5th day as compared to 68 IU/ml of phytase activity by parent on 11th day. There was difference in amount of protein secretion in these strains (Table 1). Native and SDS-gel electrophoresis of fermented broth of parent N-1 and N-79 show significant difference in protein pattern suggesting their unique nature (Fig. 1). Similar observation was reported by Chelius and Wodzinski [5] during strain improvement of A. niger for phytase production using combination of UV radiation and resistance to hygromycin B in which mutant 2DE was found to produce 3.3-fold increase in phytase activity with protein concentration in fermented broth 0.530 and 0.171 μg/μl in parent and mutant 2DE, respectively. In the present studies mutant (N-79) show 54% increase in productivity of phytase in rice bran-glucose-salt fermentation medium.
a Comparison of protein profile of extracellular culture filtrate of parent and mutants on native gel electrophoresis (8%). Lane 1 parent, lane 2 mutant N-1, lane 3 mutant N-79. Arrows a, b, c and e show the difference in proteins of parent A. niger NCIM 563 and mutant N-79 and arrow d shows the difference of parent and mutant N1. b Comparative protein profile of extra cellular culture filtrate of parent and mutants on SDS-PAGE. Lane 1 parent, lane 2 mutant N-1, lane 3 mutant N-79. Arrows a and c show the difference in protein of parent A. niger NCIM 563 and mutant N1 and arrow b shows the difference of mutant N79 with parent A. niger NCIM 563
Up scaling of phytase production (fermenter studies)
After optimizing the production of phytase under shake flask conditions it was further optimized in 3 and 14 L (New Brunswick, USA) fermenter with working volume of 1.8 and 10 L, respectively.
Three-liter fermenter
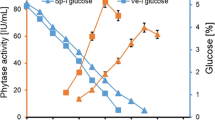
The production pattern of phytase by A. niger NCIM 563 studied using a laboratory scale fermenter (New Brunswick, USA) of 3 L capacity with a working volume of 1.8 L. The fermenter was run with aeration of 0.2 vvm and agitation (300–500 rpm), respectively. Samples were withdrawn at regular time intervals and analyzed for protein content, residual sugar and phytase activity. The pH of the fermenter was not controlled but was monitored. Initial pH of the medium was around 5.5, which decreased to pH 1.8–2.0 at the end of fermentation. Controlling the pH of the fermenter to 5.5 or at acidic side does not significantly increase or decrease the phytase activity (Data not shown). According to Vats et al. [25] phytase production by A. niger van Teighem in laboratory scale fermenter was influence by the pH of fermentation medium. They reported maximum of 141 nKat/ml phytase activity when the medium pH was maintained at pH 2.5 as compared to 17 nKat/ml units at controlled pH 5.5. Effect of various agitation speeds on phytase production indicated that maximum phytase activity of 41 IU/ml (680 nKat/ml) was obtained on 7–8th day at 400 rpm (Fig. 2). Similarly effect of various concentration of glucose in fermentation medium indicates that maximum phytase activity of 51 IU/ml (846 nKat/ml) was produced on 8th day of fermentation when glucose concentration in the medium was 4% (Fig. 3).
Fourteen-liter fermenter
From 3 L fermenter the process was further up scaled to 14 L fermenter using 1% rice bran and glucose (3.5–5%) supplemented with salts with aeration and agitation at 0.2 vvm and 400 rpm, respectively. Maximum phytase activity of 68 IU/ml (1,128 nKat/ml) and 66 IU/ml (1,095 nKat/ml) was obtained at glucose concentration (4 and 3.5%), respectively on the 10th day of fermentation (Fig. 4). Further optimization of agitation speed (400–550 rpm) in 1% rice bran–3.5% glucose–salt medium indicate that maximum phytase activity of 80 IU/ml (1,328 nKat/ml) was obtained on 10th day of fermentation at 550 rpm (Fig. 5). However, in case of A. niger van Teighem phytase production in 7 L fermenter was significantly influenced by higher agitation rate. A maximum of 203 nKat/ml phytase units were achieved at 300 rpm as compared to 91 nKat/ml at 500 rpm [25].
Stability of phytase in liquid and solid conditions
Phytase is applied in mash feed and pelleted feed. So stability of phytase enzyme in liquid form was evaluated. Effect of various additives in the liquid enzyme indicates that enzyme preparation can be stabilized in liquid form before mixing into carrier for its easy application. Phytase retains total activity without any contamination in presence of 50% methanol, ethanol, chloroform and acetone at room temperature and 8–10°C (Fig. 6a, b). Effect of various reagents indicates that fermented broth retains its total activity at room temperature by addition of penicillin and bavistin (0.1%), formalin (0.2%), sodium chloride (10%) and glycine (1 M) (Fig. 6a). Sugar alcohols were not effective in increasing the thermostability of phytase (Data not shown), even though there are several reports of increased thermo stability of various enzymes as in case of xylanase from alkalothermophilic Thermomonospora sp. [7] and protease from Bacillus cereus BG1 [8]. Addition of skimmed milk (8–12%) to phytase enzyme solution was found useful to retain the phytase activity at 70°C (Table 2). Above 70°C skimmed milk was not effective in retaining the thermo stability. Addition of 6–12% milk powder, a source of casein and calcium ions, was found to retain 76–82% of lipase activity when added to fermented broth before spray drying [1, 6]. Use of antimicrobial agents and sodium chloride has been reported to avoid contamination in fermented broth and stabilization of alkaline protease activity from Conidiobolous coronatus [14].
a Effect of various additives on stability of fermented broth (phytase) at room temperature. b Effect of various additives on stability of fermented broth (phytase) at 4ºC. 1-Chloroform (50%), 2-acetone (50%), 3-methanol (50%), 4-ethanol (50%), 5-bavistin (0.1%), 6-penicillin (0.1%), formalin (0.2%), NaCl (10%)
Release of inorganic phosphorus from poultry feed ingredients
In poultry, the feed supplied is generally digested within 2 h so to determine efficacy of phytase in poultry feed various feed ingredients were treated with phytase at 39°C for 2 h to simulate the condition of poultry stomach and the amount of Pi released was evaluated. From most of the feed ingredients 4.46–12.9 mg/g inorganic phosphorus was found to be released (Table 3).
Bioformulation for application in poultry feed
Among various carriers evaluated wheat bran and rice bran were found to retain total phytase activity in dry free flowing form. However, calcium carbonate and silica gel powders were found to loose phytase activity very rapidly (Table 4). Thus concentrated liquid phytase solution can be mixed with either wheat bran or rice bran and dried at 50°C for 2 h to remove the moisture and can be subsequently mixed with poultry feed as source of phytase. This method is more suitable and cost effective than the existing commercial phytase which is generally available in granular or powder form.
Conclusions
Earlier we have reported phytase production by A. niger NCIM 563 under solid-state and submerged fermentation conditions [2, 16, 23]. In the present studies we have successfully isolated hyper secretary mutants (N-1 and N-79) by combination of physical (UV) and chemical (EMS) mutagenesis with increase in productivity of parent strain from 6,181 IU/L per day (1,02,604 nKat/L per day) to mutant strain (N-79) to 9,523 IU/L per day (1,58,081 nKat/L per day). Even though UV mutagenesis has been reported by Chelius [5] the chemical mutagenesis by ethyl methyl sulfonate (EMS) has not reported so far for isolation of hyper secretary mutant for phytase production. We have up scaled the fermentation from shake flask to 3 L and subsequently to 14 L fermenter with reduction in fermentation time from 13 to 14 days to shake flask to 7–8 days in 14 L fermenter thus increasing the overall productivity by 54%. Studies on preservation of fermented broth for further down streaming indicate that antimicrobial agents like penicillin and bavistin along with formalin and sodium chloride could be used to stabilize the phytase activity. Thermo stabilization studies indicate100% protection of phytase activity in presence of 12% skim milk at 70°C, which will be useful for its spray drying to obtain phytase enzyme in powder form. Similarly formulation studies indicate that concentrated enzyme can be mixed with agriculture residues like wheat bran or rice bran which may be useful to supply the enzyme in form of free flowing powder. Further work on up scaling the process to 100 L fermenter with hyper secretary mutant N-79 is in progress.
References
Alloue WAM, Destain J, Amighi K, Thonart P (2007) Storage of Yarrowia lipolytica lipase after spray drying in the presence of additives. Process Biochem 42:1357–1361. doi:10.1016/j.procbio.2007.05.024
Bhavsar K, Shah P, Soni SK, Khire JM (2008) Influence of pretreatment of agriculture residues on phytase production by Aspergillus niger NCIM 563 under submerged fermentation conditions. Afr J Biotechnol 7:1101–1106
Boling SD, Douglas MW, Johnson ML, Wang X, Parsons CM, Koelkebeck KW et al (1986) Phytic acid—chemistry and application. The Pillsbury Co. Pilatus Press, Minneapolis, pp 42–44
Cao L, Wang W, Yang C, Yang Y, Diana J, Yakupitiyage A et al (2007) Application of microbial phytase in fish. Enzyme Microb Technol 40:497–507. doi:10.1016/j.enzmictec.2007.01.007
Chelius MK, Wodzinski RJ (1994) Strain improvement of Aspergillus niger for phytase production. Appl Microbiol Biotechnol 41:79–83. doi:10.1007/BF00166085
Fickers P, Ongena M, Destain J, Weekers F, Thonart P (2006) Production and down-stream processing of extracellular lipase from the yeast Yarrowia lipolytica. Enzyme Microb Technol 38:756–759
George S, Ahmad A, Rao MB (2001) A novel thermostable xylanase from Thermomonospora sp: influence of additives on themostability. Bioresour Technol 78:221–224. doi:10.1016/S0960-8524(01)00029-3
Ghorbel B, Sellami-Kamoun A, Nasari M (2003) Stability studies of protease from Bacillus cereus BG1. Enzyme Microbiol Technol 32:513–518. doi:10.1016/S0141-0229(03)00004-8
Graf E (1986) Phytic acid—Chemistry and Application. The Pillsbury Co. Pilatus Press, Minneapolis, pp 42–44
Heinohen JK, Lathi RJ (1981) A new and convenient colorimetric determination of inorganic orthophosphate and its application to the assay of inorganic pyrophosphatase. Anal Biochem 113:313–317. doi:10.1016/0003-2697(81)90082-8
Howson SJ, Davis RP (1983) Production of phytate hydrolyzing enzyme by some fungi. Enzyme Microb Technol 5:377–382. doi:10.1016/0141-0229(83)90012-1
Irvine GCJ, Cosgrove DJ (1972) Inositol phosphate phosphatase of microbiological origin: the inositol pentaphosphate products of Aspergillus ficuum phytase. J Bacteriol 112:434–438
Kim YO, Kim HK, Baeks YuJH, Oh TK (1998) Purification and properties of a thermo stable phytase from Bacillus spp. DS11. Enzyme Microbiol Technol 22:2–7. doi:10.1016/S0141-0229(97)00096-3
Laxman RS, Sonawane AP, More SV, Rao BS, Rela MV, Jogdand VV et al (2005) Optimization and scale up of production of alkaline protease from Conidiobolous coronatus. Process Biochem 40:3152–3158. doi:10.1016/j.procbio.2005.04.005
Lowry OH, Rosebrough NJ, Farr AL, Randall RL (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Mandviwala TN, Khire JM (2000) Production of high activity thermo stable phytase from thermo tolerant Aspergillus niger in solid-state fermentation. J Ind Microbiol Biotechnol 24:237–243. doi:10.1038/sj.jim.2900811
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:427–431
Mitchell DB, Vogel K, Weimann BJ, Pasamontes L, Van Loon APGM (1997) The phytase subfamily of histidine acid phosphatases: isolation of genes for two novel phytases from the fungi Aspergillus terreus and Myceliophthora thermophila. Microbiology 143:245–252
Mullaney EJ, Daly C, Ullah AB (2000) Advances in phytase research. Adv Appl Microbiol 47:157–199
Nair VC, Duvnkak Z (1991) Phytic acid content reduction in canola meal by various microorganisms in a solid-state fermentation process. Acta Biotechnol 11:211–218. doi:10.1002/abio.370110306
Reddy NR, Sathe SK, Salunkhe DK (1982) Phytases in legumes and cereals. Adv Food Res 82:1–92
Soni SK, Khire JM (2005) A process for preparation of acidic phytase. Indian Patent CSIR No. NF-180/2005
Soni SK, Khire JM (2007) Production and partial characterization of two types of phytase from Aspergillus niger NCIM 563 under submerged fermentation conditions. World J Microbiol Biotechnol 23:1585–1593. doi:10.1007/s11274-007-9404-9
Vats P, Banerjee UC (2004) Production studies and catalytic properties of phytases (myoinositolhexakisphosphate phosphohydrolases): an overview. Enzyme Microbiol Technol 35:3–14. doi:10.1016/j.enzmictec.2004.03.010
Vats P, Sahoo DK, Banerjee UC (2004) Production of phytase (myo-inositolhexakis phosphate phosphohydrolase) by Aspergillus niger van Teighem in laboratory scale fermenter. Biotechnol Prog 20:737–743. doi:10.1021/bp034095v
Vohra A, Satyanarayana T (2003) Phytases: microbial sources, production, purification and potential biotechnological applications. Crit Rev Biotechnol 23:29–60. doi:10.1080/713609297
Wodzinski RJ, Ullah AHJ (1996) Phytase. Adv Appl Microbiol 42:263–302
Acknowledgments
The authors gratefully acknowledge financial support provided by the Department of Biotechnology, New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shah, P., Bhavsar, K., Soni, S.K. et al. Strain improvement and up scaling of phytase production by Aspergillus niger NCIM 563 under submerged fermentation conditions. J Ind Microbiol Biotechnol 36, 373–380 (2009). https://doi.org/10.1007/s10295-008-0506-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-008-0506-7