Abstract
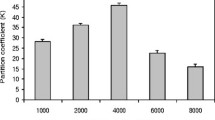
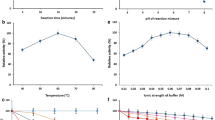
Simple, attractive and versatile technique, three-phase partitioning (TPP) was used to purify α-galactosidase from fermented media of Aspergillus oryzae. The various conditions required for attaining efficient purification of the α-galactosidase fractions were optimized. The addition of n-butanol, t-butanol, and isopropanol in the presence of ammonium sulfate pushes the protein out of the solution to form an interfacial precipitate layer between the lower aqueous and upper organic layers. The single step of three-phase partitioning, by saturating final concentration of ammonium sulfate (60%) with 1:1 t-butanol, gave activity recovery of 92% with 12-fold purification at second phase of TPP. The final purified enzyme after TPP showed considerable purification on SDS-PAGE with a molecular weight of 64 kDa. The enzyme after TPP showed improved activity in organic solvents. Results are compared with conventional established processes for the purification of α-galactosidase produced by Aspergillus oryzae and overall the proposed TPP technique resulted in 70% reduction of purification cost compared to conventional chromatographic protocols.





Similar content being viewed by others
References
Sadana A, Beelaram A (1994) Efficiency and economics of bioseparation: some case studies. Bioseparation 4:221–235
Roy I, Gupta MN (2000) Current trends in affinity base separation of proteins/enzymes. Curr Sci 78:587–591
Dennison C, Lovrein R (1997) Three phase-partitioning: concentration and purification of proteins. Protein Expr Purif 11:149–161. doi:10.1006/prep.1997.0779
Saxena L, Iyer BK, Ananthanarayan L (2007) Three phase-partitioning as a novel method for purification of ragi (Eleusine coracana) bifunctional amylase/protease inhibitor. Process Biochem 42:491–595. doi:10.1016/j.procbio.2006.09.016
Sharma A, Gupta MN (2001) Purification of pectinases by three-phase partitioning. Biotechnol Lett 23:625–1627. doi:10.1023/A:1011984517432
Dey PM, Pridham JB (1972) Biochemistry of α-galactosidase. Adv Enzymol 36:91–130
Ulezlo IV, Zaprometova OM (1982) Microbial α-Galactosidase (a review). Prikl Biokhim Mikrobiol 18:3–15
Mulimani VH, Ramalingam (1995) Enzymic hydrolysis of raffinose and stachyose present in soymilk by crude α-galactosidase from Gibberella fujikuroi. Biochem Molecul Biol Intern 36:897–905
Girigouda K, Prashanth SJ, Mulimani VH (2005) Oligosaccharides of black gram (Vigna Munga) as effected by processing methods. Plant Foods Hum Nutr 60:173–180. doi:10.1007/s11130-005-9552-3
Morton RK (1950) Separation and purification of enzymes associated with insoluble particles. Nature 166:1092–1095. doi:10.1038/1661092a0
Pike RN, Dennison C (1989) Protein fractionation by three phase-partitioning (TPP) in aqueous t-butanol mixtures. Biotechnol Bioeng 33:221–228. doi:10.1002/bit.260330213
Prashanth SJ, Mulimani VH (2005) Soymilk oligosaccharides hydrolysis by Aspergillus oryzae α-galactosidase immobilized in calcium alginate. Process Biochem 40:1199–1205. doi:10.1016/j.procbio.2004.04.011
Kulkarni DS, Kapanoor SS, Girigouda K, Kote NV, Mulimani VH (2006) Reduction of flatus inducing factors in soy milk. Biotechnol Appl Biochem 45:51–57. doi:10.1042/BA20060027
Sharma A, Sharma S, Gupta MN (2000) Purification of alkaline phosphatase from chicken intestine by three-phase partitioning and use of phenyl-Sepharose 6B in the batch mode. Bioseparation 9:155–161. doi:10.1023/A:1008195729472
Lowry AH, Rosebrough NJ, Far AL, Randall J (1950) Protein measurement with the phenol reagent. J Biol Chem 193:265
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. doi:10.1038/227680a0
Ramalingam G, Mulimani VH (2004) Purification and characterization of guar galactomannann degrading α-galactosidase form Aspergillus oryzae DR-5. J Microbiol Biotechnol 11:863–867
Kotwal SK, Gote MM, Khan IM, Khire JM (1999) Production purification and characterization of a constitutive intracellular α-galactosidase form the thermophilic fungus. Hemicola sp. J Ind Microbiol Biotechnol 23:661–667. doi:10.1038/sj.jim.2900680
Mi S, Meng K, Wang Y, Yuan T, Luo H, Yao B (2007) Molecular cloning and characterization of a novel α-galactosidase gene from Penicillium sp F63 CGMCC 1669 and expression in Pichia pastoris. Enzyme Microb Technol 1380:1373–1380. doi:10.1016/j.enzmictec.2006.10.017
Lovrein RE, Goldensoph C, Andreson P, Odegard B (1987) Three phase partitioning (TPP) via t-butanol: enzyme separation from crudes. In: Burgess R (ed) Protein Purification Micro to Macro. Marcel Dekker, New York, pp 521–533
Dennison C, Moolman L, Pillay CS, Meinesz RE (2000) t-Butanol: Nature gift for protein isolation. S Afr J Sci 96:159–160
Szamos J, Kiss E (1995) Interfacial behavior of proteins in three phase partitioning using salt containing water/tert-butanol systems. J Colloid Interface Sci 170:290–292. doi:10.1006/jcis.1995.1101
Sharma S, Gupta MN (2000) Three phase partitioning as a large scale separation method for purification of a wheat germ bifunctional protease/amylase inhibitors. Process Biochem 37:193–196. doi:10.1016/S0032-9592(01)00199-6
Gupta MN (1992) Enzyme function in organic solvents. Eur J Biochem 203:25–32. doi:10.1111/j.1432-1033.1992.tb19823.x
Singh RK, Gourinath S, Sharma S, Roy I, Gupta MN, Betzel CH et al (2001) Enhancement of enzyme activity through three phase-partitioning: crystal structure of a modified serine protease at 1.5 A° resolution. Protein Eng 14:307–313. doi:10.1093/protein/14.5.307
Roy I, Sharma A, Gupta MN (2004) Obtaining higher transesterification rates with subtilisin Carlsberg in nonaqueous media. Bioorg Med Chem Lett 14:887–889. doi:10.1016/j.bmcl.2003.12.021
Tanuja S, Srinivas ND, Gowthman MK, Raghavarao KSMS (2000) Aqueous two phase extraction coupled with ultrafiltration for purification of amyloglucosidase. Bioprocess Eng 23:63–68. doi:10.1007/s004499900123
Acknowledgments
One the author Dhananjay SK is thankful to Council of Scientific and Industrial Research (CSIR) government of India, for providing financial support in the form of Senior Research Fellowship (SRF).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dhananjay, S.K., Mulimani, V.H. Three-phase partitioning of α-galactosidase from fermented media of Aspergillus oryzae and comparison with conventional purification techniques. J Ind Microbiol Biotechnol 36, 123–128 (2009). https://doi.org/10.1007/s10295-008-0479-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-008-0479-6