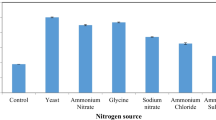
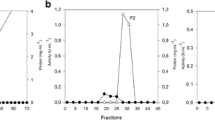
Abstract
Tomato pomace and pectin were used as the sole carbon sources for the production of polygalacturonase from a strain of Coriolus versicolor in submerged culture. The culture of C. versicolor grown on tomato pomace exhibited a peak of polygalacturonase activity (1,427 U/l) on the third day of culture with a specific activity of 14.5 U/mg protein. The production of polygalacturonase by C. versicolor grown on pectin as a sole carbon source increased with the time of cultivation, reaching a maximum activity of 3,207 U/l of fermentation broth with a specific activity of 248 U/mg protein. The levels of different isoenzymes of polygalacturonase produced during the culture growth were analysed by native PAGE. Differential chromatographic behaviour of lignocellulosic enzymes produced by C. versicolor (i.e. polygalacturonase, xylanase and laccase) was studied on immobilized metal chelates. The effect of ligand concentration, pH, the length of spacer arm and the nature of metal ion were studied for enzyme adsorption on immobilized metal affinity chromatography (IMAC). The adsorption of these lignocellulosic enzymes onto immobilized metal chelates was pH-dependent since an increase in protein adsorption was observed as the pH was increased from 6.0 to 8.0. The adsorption of polygalacturonase as well as other enzymes to immobilized metal chelates was due to coordination of histidine residues which are available at the protein surface since the presence of imidazole in the equilibration buffer abolished the adsorption of the enzyme to immobilized metal chelates. A one-step purification of polygalacturonase from C. versicolor was devised by using a column of Sepharose 6B-EPI 30-IDA-Cu(II) and purified enzyme exhibited a specific activity of about 150 U/mg protein, final recovery of enzyme activity of 100% and a purification factor of about 10. The use of short spacer arm and the presence of imidazole in equilibration buffer exhibited a higher selectivity for purification of polygalacturonase on this column with a high purification factor. The purified enzyme preparation was analysed by SDS-PAGE as well as by “in situ” detection of enzyme activity.





Similar content being viewed by others
References
Alvarado A, Pacheco-Delahaye E, Hevia P (2001) Value of a tomato byproduct as a source of dietary fiber in rats. Plant Foods Hum Nutr 56:335–348
Armisén P, Mateo C, Cortés E, Barredo JL, Salto F, Diez B, Rodéz L, Garcia JL, Fernandez-Lafuente R, Guissan JM (1999) Selective adsorption of poly-His tagged glutaryl acylase on tailor-made metal chelate supports. J Chromatogr A 848:61–70
Arnold FH (1991) Metal-affinity separations: a new dimension in protein processing. Biotechnology (N Y) 9:151–156
Bailey MJ, Biely P, Poutanen K (1992) Interlaboratory testing of methods for assay of xylanases activity. J Biotechnol 23:257–270
Bisswanger H (1992) Practical enzymology. Wiley–VCH, New York
Blandino A, Iqbalsyah T, Pandiella SS, Cantero D, Webb C (2002) Polygalacturonase production by Aspergillus awamori on wheat in solid-state fermentation. Appl Microbiol Biotechnol 58:164–169
Bloom H, Beier H, Gross HS (1987) Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8:248–254
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Camperi SA, Auday RM, del Canizo AN, Cascote O (1996) Study of variables involved in fungal pectic enzyme fractionation by Immobilized Metal Ion Affinity Chromatography. Process Biochem 31:81–87
Camperi SA, Grasselli M, Cascone O (2000) High-speed pectic enzyme fractionation by immobilised metal ion affinity membranes. Bioseparation 9:173–177
Camperi SA, Grasselli M, Smolko EE, Cascone O (2004) Preparation and characterisation of immobilised metal ion hollow-fibre polysulphone membranes: their application in high-speed pectic enzyme fractionation. Process Biochem 39:1017–1024
Crotti LB, Jabor VA, Chellegatti MA, Fonseca MJ, Said S (1999) Studies of pectic enzymes produced by Talaromyces flavus in submerged and solid substrate cultures. J Basic Microbiol 39:227–35
Del Valle M, Cámara M, Torija M-E (2006) Chemical characterization of tomato pomace. J Sci Food Agric 86:1232–1236
Federici L, Caprari C, Mattei B, Savino C, Di Matteo A, De Lorenzo G, Cervone F, Tsernoglou D (2001) Structural requirements of endopolygalacturonase for the interaction with PGIP (polygalacturonase-inhibiting protein). Proc Natl Acad Sci USA 98:13425–13430
Fett WF, Wijey C, Moreau RA, Osman SF (2000) Production of cutinolytic esterase by filamentous bacteria. Lett Appl Microbiol 31:25–29
Galiotou-Panayotou M, Kapantai M, Kalantzi O (1997) Growth conditions of Aspergillus sp. ATHUM-3482 for polygalacturonase production. Appl Microbiol Biotechnol 47:425–429
Hames BD (1981) An introduction to polyacrylamide gel electrophoresis. In: Hames BD, Rickwood D (eds) Gel electrophoresis of proteins. IRL Press, Oxford, pp 1–86
Hours RA, Voget CE, Ertola RJ (1988) Some factors affecting pectinase production from apple pomace in solid-state-cultures. Biol Waste 24:147–157
Jayani RS, Saxena S, Gupta R (2005) Microbial pectinolytic enzymes: a review. Process Biochem 40:2931–2944
Knoblich M, Anderson B, Latshaw D (2005) Analyses of tomato peel and seed byproducts and their use as a source of carotenoids. J Sci Food Agric 85:1166–1170
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Levin L, Forchiassin F (1998) Culture conditions for the production of pectinolytic enzymes by the white-rot fungus Coriolus troggi on a laboratory scale. Acta Biotechnol 18:157–166
Martins S, Andrade J, Karmali A, Serralheiro ML (2006a) Screening of suitable immobilized metal chelates for adsorption of monoclonal antibodies against mutant amidase from Pseudomonas aeruginosa. J Mol Recognit 19:340–347
Martins S, Andrade J, Karmali A, Serralheiro ML (2006b) Immobilized metal affinity chromatography of immunoglobulin M against mutant amidase from Pseudomonas aeruginosa. Mol Biotechnol 33:103–114
Mohamed SA, Farid NM, Hossiny EN, Bassuiny RI (2006) Biochemical characterization of an extracellular polygalacturonase from Trichoderma harzianum. J Biotechnol 127:54–64
Morag E, Bayer EA, Lamed R (1990) Relationship of cellulosomal and noncellulosomal xylanases of Clostridium thermocellum to cellulose-degrading enzymes. J Bacteriol 172:6098–6105
Pacheco V, Karmali A (1998) Chromatographic behaviour of glucose 1- and 2- oxidases from fungal strains on immobilized metal chelates. J Ind Microbiol Biotechnol 21:57–64
Patil SR, Dayanand A (2006) Production of pectinase from deseeded sunflower head by Aspergillus niger in submerged and solid-state conditions. Bioresour Technol 97:2054–2058
Rawashdeh R, Saadoun I, Mahasneh A (2005) Effect of cultural conditions on xylanase production by Streptomyces sp. (strain lb 24D) and its potential to utilize tomato pomace. Afr J Biotechnol 4:251–255
Ried JL, Collmer A (1985) Activity stain for rapid characterization of pectic enzymes in isoelectric focusing and sodium dodecyl sulphate-polyacrylamide gels. Appl Environ Microbiol 50:615–622
Gummadi SN, Panda T (2003) Purification and biochemical properties of microbial pectinases: a review. Process Biochem 38:987–996
Shanley NA, van den Broek LAM, Voragen AGJ, Coughlan MP (1993) Isolation and characterization of an endopolygalacturonase from Phanerochaete chrysosporium. J Biotechnol 28:179–197
Todd RJ, Johnson D, Arnold FH (1994) Multiple-site binding interactions in metal affinity chromatography. I: Equilibrium binding of engineered histidine-containing cytochromes c. J Chromatogr A 662:13–26
Van Santen Y, Benen JAE, Schroter K-H, Kalk KH, Armand S, Visser J, Dijkstra BW (1999) 1.68-A crystal structure of endopolygalacturonase II from Aspergillus niger and identification of active site residues by site-directed mutagenesis. J Biol Chem 274:30474–30480
van Pouderoyen G, Snijder H, Benen J, Dijkstra BW (2003) Structural insights into the processivity of endopolygalacturonase I from Aspergillus niger. Febs Lett 554:462–466
White CA, Kennedy JF (1986) Oligosaccharides. In: Chaplin M, Kennedy JF (eds) Carbohydrate analysis: a practical approach. IRL Press, Oxford
Yip TT, Hutchens TW (1994) Immobilized metal ion affinity chromatography. Mol Biotechnol 1:151–164
Acknowledgments
MRF is grateful to Évora University for financial support and the authors thank Fundação para a Ciência e Tecnologia for partial financial support (Research Unit 702, POCTI/EQU/48812/2002) as well as Instituto Politécnico de Lisboa (Research Grant No. 25/2003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
do Rosário Freixo, M., Karmali, A. & Arteiro, J.M. Production of polygalacturonase from Coriolus versicolor grown on tomato pomace and its chromatographic behaviour on immobilized metal chelates. J Ind Microbiol Biotechnol 35, 475–484 (2008). https://doi.org/10.1007/s10295-008-0305-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-008-0305-1