Abstract
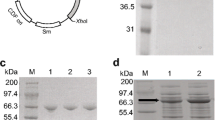
A tyrosine ammonia-lyase (TAL) enzyme from the photosynthetic bacterium Rhodobacter sphaeroides (RsTAL) was identified, cloned and functionally expressed in Escherichia coli, where conversion of tyrosine to p-hydroxycinnamic acid (pHCA) was demonstrated. The RsTAL enzyme is implicated in production of pHCA, which serves as the cofactor for synthesis of the photoactive yellow protein (PYP) in photosynthetic bacteria. The wild type RsTAL enzyme, while accepting both tyrosine and phenylalanine as substrate, prefers tyrosine, but a serendipitous RsTAL mutant identified during PCR amplification of the RsTAL gene, demonstrates much higher preference for phenylalanine as substrate and deaminates it to produces cinnamic acid. Sequence analysis showed the presence of three mutations: Met4 → Ile, Ile325 → Val and Val409 → Met in this mutant. Sequence comparison with Rhodobacter capsulatus TAL (RcTAL) shows that Val409 is conserved between RcTAL and RsTAL. Two single mutants of RsTAL, Val409 → Met and Val 409 → Ile, generated by site-directed mutagenesis, demonstrate greater preference for phenylalanine compared to the wild type enzyme. Our studies illustrate that relatively minor changes in the primary structure of an ammonia-lyase enzyme can significantly affect its substrate specificity.


Similar content being viewed by others
References
Abell CW, Shen RS (1987) Phenylalanine ammonia-lyase from yeast Rhodotorula glutinis. Methods Enzymol 142:242–248
Berner M, Krug D, Bihlmaier C, Vente A, Muller R, Bechthold A (2006) Genes and enzymes involved in caffeic acid biosynthesis in the actinomycete Saccharothrix espanaensis. J Bacteriol 188:2666–2673
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Calabrese J, Jordan DB, Boodhoo A, Sariaslani S, Vannelli T (2004) Crystal structure of phenylalanine ammonia lyase from Rhototorula glutinis. Biochemistry 43:11403–11416
Gatenby AA, Sariaslani FS, Tang X, Qi WW, Vanelli T (2002) Bioproduction of hydroxycinnamic acid. US Patent 6368837
Hermes JD, Weiss PM Cleland WW (1985) Use of nitrogen-15 and detuerium isotope effects to determine the chemical mechanism of phenylalanine ammonia-lyase. Biochemistry 24:2959–2967
Hoff WD, Dux P, Hard K, Devreese B, Nugteren-Roodzat I M, Crielaard W, Boelens R, Kaptein R, Van Beeumen J, Hellingwerf KJ (1994) Thiol ester-linked p-coumaric acid as a new photoactive prosthetic group in a protein with rhodopsin-like photochemistry. Biochemistry 33:13959–13962
Huang L, Xue Z (2006) DNA and amino acid sequence of a tyrosine ammonia lyase from the bacterium Rhodobacter sphaeroides. US Patent No. 7,067,302
Kort R, Vonk H, Xu X, Hoff WD, Crielaard W, Hellingwerf KJ (1996) Evidence for trans-cis isomerization of the p-coumaric acid chromophore as the photochemical basis of the photocycle of photoactive yellow protein. FEBS Lett 382:73–78
Kyndt JA, Meyer TE, Cusanovich MA, Meyer JJ, Van Beeumen JJ (2002) Characterization of a bacterial tyrosine ammonia lyase, a biosynthetic enzyme for the photoactive yellow protein. FEBS Lett 512:240–244
Louie GV, Bowman ME, Moffitt MC, Baiga TJ, Moore BS, Noel JP (2006) Structural determinants and modulation of substrate specificity in phenylalanine–tyrosine ammonia lyases. Chem Biol 13:1327–1338
Moffitt MC, Louie GV, Bowman ME, Pence J, Noel JP, Moore BS (2007) Discovery of two cyanobacterial phenylalanine ammonia lyases: kinetic and structural characterization. Biochemistry (published online). doi:10.1021/bi061774g
Ritter H, Schulz GE (2004) Structural basis for the entrance into the phenylpropanoid metabolism catalyzed by phenylalanine ammonia-lyase. Plant Cell 16:3426–3436
Rosler J, Krekel N, Amrhein N, Schmid J (1997) Maize phenylalanine ammonia-lyase has tyrosine ammonia-lyase activity. Plant Physiol 113:175–179
Schwede TF, Retey J, Schulz GE (1999) Crystal structure of histidine ammonia-lyase revealing a novel polypeptide modification as the catalytic electrophile. Biochemistry 38:5355–5361
Watts KT, Lee PC, Schmidt-Dannert C (2004) Exploring recombinant flavonoid biosynthesis in metabolically engineered Escherichia coli. Chembiochem 5:500–507
Wang L, Gamez A, Sarkissian CN, Straub M, Patch MG, Han GW, Striepeke S, Fitzpatrick P, Scriver CR, Stevens RC (2005) Structure-based chemical modification strategy for enzyme replacement treatment of phenylketonuria. Mol Gen Metab 86:134–140
Watts KT, Mijts BN, Lee PC, Manning AJ, schmidt-Dannert C (2006) Discovery of a substrated selectivity switch in tyrosine ammonia-lyase, a member of the aromatic amino acid lyase family. Chem Biol 13:1317–1326
Xiang L, Moore BS (2002) Inactivation, complementation, and heterologous expression of encP, a novel bacterial phenylalanine ammonia-lyase gene. J Biol Chem 277:32505–32509
Xie A, Hoff WD, Kroon AR, Hellingwerf KJ (1996) Glu46 donates a proton to the 4-hydroxycinnamate anion chromophore during the photocycle of photoactive yellow protein. Biochemistry 35:14671–14678
Acknowledgment
We thank Joseph Calabrese, and Anthony Gatenby for helpful discussions and review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xue, Z., McCluskey, M., Cantera, K. et al. Identification, characterization and functional expression of a tyrosine ammonia-lyase and its mutants from the photosynthetic bacterium Rhodobacter sphaeroides . J Ind Microbiol Biotechnol 34, 599–604 (2007). https://doi.org/10.1007/s10295-007-0229-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-007-0229-1