Abstract
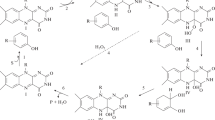
Polycyclic aromatic hydrocarbon (PAH) quinone reductase (PQR) and catechol-O-methyltransferase (COMT), from the PAH-degrading Mycobacterium vanbaalenii PYR-1, were demonstrated to be constitutive enzymes located in the soluble fraction of cell extracts. PQR activities for the reduction of 9,10-phenanthrenequinone and 4,5-pyrene- quinone were 1.40±0.13 and 0.12±0.01 μmol min−1 mg-protein−1, respectively. The exogenous catechols alizarin, anthrarobin, 2,3-dihydroxynaphthalene and esculetin inhibited PQR activity. Anthrarobin (100 μM) and esculetin (100 μM) inhibited 4,5-pyrenequinone reduction by 64–92%. COMT was involved in the O-methylation of 1,2-dihydroxyphenanthrene to form 1-methoxy-2-hydroxyphenanthrene and 1,2-dimethoxyphenanthrene. Both pyrene and 1-hydroxypyrene were metabolized by M. vanbaalenii PYR-1 to form 1-methoxypyrene, 1-methoxy-2-hydroxypyrene, 1-hydroxy-2-methoxypyrene and 1,2-dimethoxypyrene. Among the catechols tested, anthrarobin showed the highest COMT activity (1.06±0.04 nmol/30 min−1 mg-protein−1). These results suggest that the PQR and COMT activities of M. vanbaalenii PYR-1 may play an important role in the detoxification of PAH catechols.






Similar content being viewed by others
References
Bolton JL, Michael MA, Penning TM, Dryhurst G, Monks TJ (2000) Role of quinones in toxicity. Chem Res Toxicol 13:135–160
Brezna B, Khan AA, Cerniglia CE (2003) Molecular characterization of dioxygenases from polycyclic aromatic hydrocarbon-degrading Mycobacterium spp. FEMS Microbiol Lett 223:177–183
Cerniglia CE (1993) Biodegradation of polycyclic aromatic hydrocarbons. In: Rosenberg E (ed) Microorganisms to combat pollution. Kluwer, Dordrecht, pp 227–244
Cheung P, Kinkle B (2001) Mycobacterium diversity and pyrene mineralization in petroleum-contaminated soils. Appl Environ Microbiol 67:2222–2229
Heitkamp MA, Franklin W, Cerniglia CE (1988) Microbial metabolism of polycyclic aromatic hydrocarbons: isolation and characterization of a pyrene-degrading bacterium. Appl Environ Microbiol 54:2549–2555
Heitkamp MA, JP Freeman, DW Miller, Cerniglia CE (1988) Pyrene degradation by a Mycobacterium sp.: identification of ring oxidation and ring fission products. Appl Environ Microbiol 54:2556–2565
Kazunga C, Aitken MD (2000) Products from the incomplete metabolism of pyrene by polycyclic aromatic hydrocarbon-degrading bacteria. Appl Environ Microbiol 66:1917–1922
Kelley I, Cerniglia CE (1995) Degradation of a mixture of high-molecular-weight polycyclic aromatic hydrocarbons by a Mycobacterium strain PYR-1. J Soil Contam 4:44–91
Kelley I, Freeman JP, Evans FE, Cerniglia CE (1993) Identification of metabolites from the degradation of fluoranthene by Mycobacterium sp. strain PYR-1. Appl Environ Microbiol 59:800–806
Khan AA, Kim S-J, Paine DD, Cerniglia CE (2002) Classification of a polycyclic aromatic hydrocarbon-metabolizing bacterium, Mycobacterium sp. strain PYR-1, as Mycobacterium vanbaalenii sp. nov. Int J Syst Evol Microbiol 52:1997–2002
Kim Y-H, Engesser K-H, Cerniglia CE (2003) Two polycyclic aromatic hydrocarbon o-quinone reductases from a pyrene-degrading Mycobacterium. Arch Biochem Biophys 416:209–217
Kulakov L, Allen CCR, Lipscomb DA, Larkin MJ (2000) Cloning and characterization of a novel cis-napthalene dihydrodiol dehydrogenase gene (narB) from Rhodococcus sp. NCIMB12038. FEMS Microbiol Lett 182:327–331
McLellan S, Warshawsky D, Shann J (2002) The effect of polycyclic aromatic hydrocarbons on the degradation of benzo[a]pyrene by Mycobacterium sp. strain RJGII-135. Environ Toxicol Chem 21:253–259
Molina M, Araujo R, Hodson R (1999) Cross-induction of pyrene and phenanthrene in a Mycobacterium sp. isolated from polycyclic aromatic hydrocarbon contaminated river sediments. Can J Microbiol 45:520–529
Moody JD, Freeman JP, Doerge DR, Cerniglia CE (2001) Degradation of phenanthrene and anthracene by cell suspensions of Mycobacterium sp. strain PYR-1. Appl Environ Microbiol 67:1476–1483
Moody JD, Fu PP, Freeman JP, Cerniglia CE (2003) Regio- and stereoselective metabolism of 7,12-dimethylbenz[a]anthracene by Mycobacterium vanbaalenii PYR-1. Appl Environ Microbiol 69:3924–3931
Moody JD, Freeman JP, Fu PP, Cerniglia CE (2004) Degradation of benzo[a]pyrene by Mycobacterium vanbaalenii PYR-1. Appl Environ Microbiol 70:340–345
Nappi AJ, Vass E (1998) Hydroxyl radical formation via iron-mediated Fenton chemistry is inhibited by methylated catechols. Biochim Biophys Acta 1425:159–167
Nappi AJ, Vass E, Collins MA (1999) Contrasting effects of catecholic and O-methylated tetrahydroisoquinolines on hydroxyl radical production. Biochim Biophys Acta 1434:64–73
Narro ML, Cerniglia CE, Van Baalen C, Gibson DT (1992) Metabolism of phenanthrene by the marine cyanobacterium Agmenellum quadruplicatum PR-6. Appl Environ Microbiol 58:1351–1359
Pangrekar J, Kandaswami C, Kole P, Kumar S, Sikka HC (1995) Comparative metabolism of benzo[a]pyrene, chrysene and phenanthrene by brown bullhead liver microsomes. Mar Environ Res 39:51–55
Penning TM (1993) Dihydrodiol dehydrogenase and its role in polycyclic aromatic hydrocarbon metabolism. Chem Biol Interact 89:1–34
Rehmann K, Noll H, Steinberg C, Kettrup A (1998) Pyrene degradation by Mycobacterium sp. strain KR2. Chemosphere 36:2977–2992
Rehmann K, Hertkorn N, Kettrup A (2001) Fluoranthene metabolism in Mycobacterium sp. strain KR20: identification of pathway intermediates during degradation and growth. Microbiology 147:2783–2794
Sambrook J, Russell DW (2001) Molecular cloning, a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Schneider J, Grosser R, Jayasimhulu K, Xue W, Warshawsky D (1996) Degradation of pyrene, benz[a]anthracene, and benzo[a]pyrene by Mycobacterium sp. strain RJGII-135, isolated from a former coal gasification site. Appl Environ Microbiol 62:13–19
Vila J, López Z, Sabaté J, Minguillón C, Solanas AM, Grifoll M (2001) Identification of a novel metabolite in the degradation of pyrene by Mycobacterium sp. strain AP1: actions of the isolate on two- and three-ring polycyclic aromatic hydrocarbons. Appl Environ Microbiol 67:5497–5505
Willumsen P, Karlson U, Stackebrandt E, Kroppenstedt R (2001) Mycobacterium frederiksbergense sp. nov., a novel polycyclic aromatic hydrocarbon-degrading Mycobacterium species. Int J Syst Evol Microbiol 51:1715–1722
Wunder T, Marr J, Kremer S, Sterner O, Anke H (1997) 1-Methoxypyrene and 1,6-methoxypyrene: two novel metabolites in fungal metabolism of polycyclic aromatic hydrocarbons. Arch Microbiol 167:310–316
Zhu BT, Conney AH (1998) Is 2-methoxyestradiol an endogenous estrogen metabolite that inhibits mammary carcinogenesis? Cancer Res 58:2269–2277
Zhu BT, Conney AH (1998) Functional roles of estrogen metabolism in target cells: Review and perspectives. Carcinogenesis 19:1–27
Zhu BT, Patel UK, Cai MX, Conney AH (2000) O-Methylation of tea polyphenols catalyzed by human placental cytosolic catechol-O-methyltransferase. Drug Metab Dispos 28:1024–1030
Acknowledgements
The authors thank John B. Sutherland for critical review of the manuscript, and Diana Mathews for clerical assistance. This research was supported by the Postgraduate Research Participation Program at the National Center for Toxicological Research administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the United States Department of Energy and the Food and Drug Administration.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, YH., Moody, J.D., Freeman, J.P. et al. Evidence for the existence of PAH-quinone reductase and catechol-O-methyltransferase in Mycobacterium vanbaalenii PYR-1. J IND MICROBIOL BIOTECHNOL 31, 507–516 (2004). https://doi.org/10.1007/s10295-004-0178-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-004-0178-x