Abstract
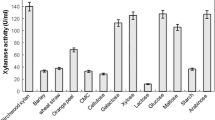
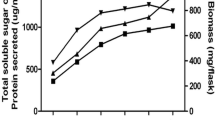
Microbial xylanases that are thermostable, active at alkaline pH and cellulase-free are generally preferred for biobleaching of paper pulp. We screened obligate and facultative marine fungi for xylanase activity with these desirable traits. Several fungal isolates obtained from marine habitats showed alkaline xylanase activity. The crude enzyme from NIOCC isolate 3 (Aspergillus niger), with high xylanase activity, cellulase-free and unique properties containing 580 U l−1 xylanase, could bring about bleaching of sugarcane bagasse pulp by a 60 min treatment at 55°C, resulting in a decrease of ten kappa numbers and a 30% reduction in consumption of chlorine during bleaching. The culture filtrate showed peaks of xylanase activity at pH 3.5 and pH 8.5. When assayed at pH 3.5, optimum activity was detected at 50°C, with a second peak of activity at 90°C. When assayed at pH 8.5, optimum activity was seen at 80°C. The crude enzyme was thermostable at 55°C for at least 4 h and retained about 60% activity. Gel filtration of the 50–80% ammonium sulphate-precipitated fraction of the crude culture filtrate separated into two peaks of xylanase with specific activities of 393 and 2,457 U (mg protein)−1. The two peaks showing xylanase activity had molecular masses of 13 and 18 kDa. Zymogram analysis of xylanase of crude culture filtrate as well as the 50–80% ammonium sulphate-precipitated fraction showed two distinct xylanase activity bands on native PAGE. The crude culture filtrate also showed moderate activities of β-xylosidase and α-l-arabinofuranosidase, which could act synergistically with xylanase in attacking xylan. This is the first report showing the potential application of crude culture filtrate of a marine fungal isolate possessing thermostable, cellulase-free alkaline xylanase activity in biobleaching of paper pulp.









Similar content being viewed by others
References
Ali M, Sreekrishnan TR (2001) Aquatic toxicity from pulp and paper mill effluents: a review. Adv Environ Res 5:175–196
Anonymous (1988) Kappa number of pulp-T 236- cm- 85. In: TAPPI test methods, vol 1. TAPPI Press, Atlanta, pp 1–3
Anthony T, Chandra Raj K, Rajendran A, Gunasekaran P (2003) High molecular weight cellulase-free xylanase from alkali-tolerant Aspergillus fumigatus AR1. Enzyme Microb Technol 32:647–654
Biswas SR, Jana SC, Mishra AK, Nanda G (1990) Production, purification and characterization of xylanase from a hyperxylanolytic mutant of Aspergillus ochraceus. Biotechnol Bioeng 35:244–251
Casimir-Schenkel J, Davis S, Fiechter A, Gygin B, Murray E, Perrolax J, Zimmermann W (1995) Pulp bleaching with thermostable xylanase of Thermomonospora fusca. US Patent 5407827, 18 April 1995
Cundell AM, Brown MS, Stanford R, Mitchell R (1979) Microbial degradation of Rhizophora mangle leaves immersed in the sea. Estuarine Coastal Shelf Sci 9:281–286
Domsch KH, Gams W, Anderson TH (1980) Compendium of soil fungi. Academic, London
Duarte JC, Maximo C, Dias A, Costa-Ferreira M, Vasconcelos L, Morgado MJ (1993) Biobleaching of kraft pulp by Aspergillus niger xylanolytic enzymes. In: Proceedings of the symposium on Lignin: biodegradation and transformation. Lisbon, pp 211–214
Eriksson KE (1993) Concluding remarks: where do we stand and where are we going? Lignin biodegradation and practical utilization. J Biotechnol 30:149–158
Fell JW, Newell SY (1981) Role of fungi in carbon nitrogen immobilization in coastal marine plant litter systems. In: Wicklow DT, Carroll GC (eds) The fungal community. Dekker, New York, pp 665–678
Frederick MM, Kiang CH, Frederick JR, Reilly PJ (1985) Purification and characterization of endo-xylanases from Aspergillus niger. I. Two isozymes active on xylan backbones near branch points. Biotechnol Bioeng 27:525–532
Gessesse A (1998) Purification and properties of two thermostable alkaline xylanases from an alkaliphilic Bacillus sp. Appl Environ Microbiol 64:3533–3535
Ghosh TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268
Haack S (1992) Description of a new species of Cytophaga and characterization of its xylan-degrading enzyme system. Ph.D. thesis. Michigan State University
Jensen PR, Fenical W (1999) Exploring marine fungi as a source of novel pharmaceutical leads. In: Abstracts of the 7th international marine and fresh water mycology symposium, Hong Kong, p 55
Kang MK, Maeng PJ, Rhee YA (1996) Purification and characterization of two xylanases from alkalophilic Cephalosporium sp. Strain RYM-202. Appl Environ Microbiol 62:3480–3482
Kohlmeyer J, Kohlmeyer E (1979) Marine mycology, the higher fungi. Academic, New York, p 690
Leathers TD (1986) Color variants of Aureobasidium pullulans overproduce xylanase with extremely high specific activity. Appl Environ Microbiol 52:1026–1030
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin Phenol reagent. J Biol Chem 193:265–275
Morales PJ, Madarro J, Perez-Gonzalez A, Sendra JM, Pinaga F, Flors A (1993) Purification and characterization of alkaline xylanases from Bacillus polymyxa. Appl Environ Microbiol 59:1376–1382
Nakamura S, Wakabayashi K, Nakai, R, Aono R, Horikoshi K (1993) Purification and some properties of an alkaline xylanase from alkaliphilic Bacillus sp. strain 41 —1. Appl Environ Microbiol 59:2311–2316
Raghukumar C, Raghukumar S, Chinnaraj A, Chandramohan D, D’Souza TM, Reddy CA (1994) Laccase and other lignocellulose modifying enzymes of marine fungi isolated from the coast of India. Bot Mar 37:515–523
Raghukumar C, D’Souza TM, Thorn RG, Reddy CA (1999) Lignin-modifying enzymes of Flavodon flavus, a basidiomycete isolated from a coastal marine environment. Appl Environ Microbiol 65:2103–2111
Raghukumar C, Muraleedharan UD (2000) A process for production of xylan-degrading enzymes. Indian Patent No. NF 30/2000, 31 March 2000
Raghukumar S, Sharma S, Raghukumar C, Sathe-Pathak V, Chandramohan D (1994) Thraustochytrid and fungal component of marine detritus. IV. Laboratory studies on decomposition of leaves of the mangrove Rhizophora apiculata Blume. J Exp Mar Biol Ecol 175:227–242
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Smith DC, Wood TM (1991) Xylanase production by Aspergillus awamori: development of a medium and optimization of the fermentation parameters for the production of extracellular xylanase and β-xylosidase while maintaining low protease production. Biotechnol Bioeng 38:883–890
Srinivasan MC, Rele MV (1995) Cellulase-free xylanases from microorganisms and their application to paper and pulp biotechnology: an overview. Indian J Microbiol 35:93–101
Wizani W, Esterbauer H, Gomes J (1993) Preparation of xylanase by cultivating Thermomyces lanuginosus DSM 5826 in a medium containing corn cobs. US Patent No. 5183753,02 February 1993
Wong KK, Tan Y, Saddler JN (1988) Multiplicity of β-1,4-xylanase in microorganisms: function and applications. Microbiol Rev 52:305–317
Acknowledgements
We thank Ms. Gauri Rivonkar and Ms. Shilpa Kamat for the excellent laboratory assistance rendered at N.I.O. Our grateful thanks to Pudumjee Paper Mills Ltd., Pune, India and Seshasayee Paper Mills, Chennai for conducting bleaching trials with our enzyme. This is NIO’s contribution No. 3927.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raghukumar, C., Muraleedharan, U., Gaud, V.R. et al. Xylanases of marine fungi of potential use for biobleaching of paper pulp. J IND MICROBIOL BIOTECHNOL 31, 433–441 (2004). https://doi.org/10.1007/s10295-004-0165-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-004-0165-2