Abstract
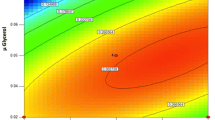
This paper provides an approach for optimizing the cell density (Xc) and dilution rate (D) in a chemostat for a Pichia pastoris continuous fermentation for the extracellular production of a recombinant protein, interferon τ (INF-τ). The objective was to maximize the volumetric productivity (Q, mg INF-τ l−1 h−1), which was accomplished using response surface methodology (RSM) to model the response of Q as a function of Xc and D within the ranges 150≤ Xc ≤450 g cells (wet weight) l−1 and 0.1 μm≤D≤0.9 μm (μm=0.0678 h−1, the maximum specific growth rate obtained from a fed-batch phase controlled with a methanol sensor). The methanol and medium feed rates that resulted in the desired Xc and D were determined based on the mass balance. From the RSM model, the optimal Xc and D were 328.9 g l−1 and 0.0333 h−1 for a maximum Q of 2.73 mg l−1 h−1. The model of specific production rate (ρ, mg INF-τ g−1 cells h−1) was also established and showed the optimal Xc=287.7 g l−1 and D=0.0361 h−1 for the maximum ρ (predicted to be 8.92×10−3 mg−1 g−1 h−1). The methanol specific consumption rate (ν, g methanol g−1 cells h−1) was calculated and shown to be independent of the cell density. The relationship between ν and μ (specific growth rate) was the same as that discovered from fed-batch fermentations of the same strain. The approach developed in this study is expected to be applicable to the optimization of continuous fermentations by other microorganisms.



Similar content being viewed by others
References
Alexenko AP, Leaman DW, Li J, Roberts RM (1997) The antiproliferative and antiviral activities of IFN-τ variants in human cells. J Interferon Cytokine Res 17:769–779
Curvers S, Brixius P, Klauser T, Thoemmes J, Weuster-Botz D, Takors R, Wandrey C (2001) Human chymotrypsinogen B production with Pichia pastoris by integrated development of fermentation and downstream processing. Part 1. Fermentation. Biotechnol Prog 17:495–502
Digan ME, Lair SV, Brierley RA, Siegel RS, Williams ME, Ellis SB, Kellaris PA, Provow SA, Craig WS, Velicelebi G, Harpold MM, Thill GP (1989) Continuous production of a novel lysozyme via secretion from the yeast, Pichia pastoris. Biotechnology 7:160–164
Goodrick JC, Xu M, Finnegan R, Schilling BM, Schiavi S, Hoppe H, Wan NC (2001) High-level expression and stabilization of recombinant human chitinase produced in a continuous constitutive Pichia pastoris expression system. Biotechnol Bioeng 74:492–497
Johnson TM, Holaday SK, Sun Y, Subramaniam PS, Johnson HM, Krishna NR (1999) Expression, purification, and characterization of interferon-τ produced in Pichia pastoris grown in a minimal medium. J Interferon Cytokine Res 19:631–636
Khan OA, Jiang H, Subramaniam PS, Johnson HM, Dhib-Jalbut SS (1998) Immunomodulating functions of recombinant ovine interferon τ: potential for therapy in multiple sclerosis and autoimmune disorders. Mult Scler 4:63–69
Myers RH, Montgomery DC (1995) Response surface methodology: process and product optimization using designed experiments. Wiley, New York
Sinha J, Plantz BA, Zhang W, Gouthro M, Schlegel VL, Liu C-P, Meagher MM (2003) Improved production of IFN-τ by Mut+ strain of Pichia pastoris using an optimized methanol feed profile. Biotechnol Prog 19:794–802
Zhang W, Bevins MA, Plantz BA, Smith LA, Meagher MM (2000) Modeling Pichia pastoris growth on methanol and optimizing the production of a recombinant protein, the heavy-chain fragment C of botulinum neurotoxin, serotype A. Biotechnol Bioeng 70:1–8
Zhang W, Potter KJ, Plantz BA, Schlegel VL, Smith LA, Meagher MM (2003) Pichia pastoris fermentation with mixed-feeds of glycerol and methanol: growth kinetics and production improvement. J Ind Microbiol Biotechnol 30:210–215
Zhang W, Smith LA, Meagher MM (2004) Maximizing secreted production of recombinant proteins in Pichia pastoris fed-batch fermentation. Biotechnol Prog (submitted)
Acknowledgements
We thank Carlee Rastede, Priya Nataraj and Mike Dux (undergraduate students at Department of Chemical Engineering, University of Nebraska–Lincoln; UNL) for running the fermentations; and we thank Sheila Bart and other staff in the QC Division of the Biological Process Development Facility at UNL for the IFN-τ assay. Pepgen Co. (Alameda, Calif., USA) provided the P. pastoris strain.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, W., Liu, CP., Inan, M. et al. Optimization of cell density and dilution rate in Pichia pastoris continuous fermentations for production of recombinant proteins. J IND MICROBIOL BIOTECHNOL 31, 330–334 (2004). https://doi.org/10.1007/s10295-004-0155-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-004-0155-4