Abstract
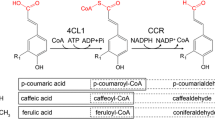
Chalcones, the central precursor of flavonoids, are synthesized exclusively in plants from tyrosine and phenylalanine via the sequential reaction of phenylalanine ammonia-lyase (PAL), cinnamate-4-hydroxylase (C4H), 4-coumarate:coenzyme A ligase (4CL) and chalcone synthase (CHS). Chalcones are converted into the corresponding flavanones by the action of chalcone isomerase (CHI), or non-enzymatically under alkaline conditions. PAL from the yeast Rhodotorula rubra, 4CL from an actinomycete Streptomyces coelicolor A3(2), and CHS from a licorice plant Glycyrrhiza echinata, assembled as artificial gene clusters in different organizations, were used for fermentation production of flavanones in Escherichia coli. Because the bacterial 4CL enzyme attaches CoA to both cinnamic acid and 4-coumaric acid, the designed biosynthetic pathway bypassed the C4H step. E. coli carrying one of the designed gene clusters produced about 450 μg naringenin/l from tyrosine and 750 μg pinocembrin/l from phenylalanine. The successful production of plant-specific flavanones in bacteria demonstrates the usefulness of combinatorial biosynthesis approaches not only for the production of various compounds of plant and animal origin but also for the construction of libraries of "unnatural" natural compounds.



Similar content being viewed by others
References
Alekel DL, St Germain A, Pererson CT, Hanson KB, Stewart JW, Toda T (2000) Isoflavone-rich soy protein attenuates bone loss in the lumber spine of perimenopausal women. Am J Clin Nutr 72:844–852
Baedeker M, Schulz GE (1999) Overexpression of a designed 2.2 kb gene of eukaryotic phenylalanine ammonia-lyase in E. coli. FEBS Lett 457:57–60
Becker-André M, Schulze-Lefert P, Hahlbrock K(1991) Structural comparison, modes of expression, and putative cis-acting elements of the two 4-coumarate:CoA ligase genes in potato. J Biol Chem 266:8551–8559
Bednar RA (1990) Reactivity and pH dependence of thiol conjugation to N-ethylmaleimide: detection of a conformational change in chalcone isomerase. Biochemistry 29:3684–3690
Bednar RA, Fried WB, Lock YW, Pramanik B (1989) Chemical modification of chalcone isomerase by mercurials and tetrathionate. J Biol Chem 264:14272–14276
Bentley SD, Chater KF, Cerdeno-Tarraga AM, Challis GL, Thomson NR, James KD, Harris DE, Quail MA, Kieser H, Harper D, Bateman A, Brown S, Chandra G, Chen CW, Collins M, Cronin A, Fraser A, Goble A, Hidalgo J, Hornsby T, et al (2002) Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141–147
Bingham SA, Atkinson C, Liggins J, Bluck L, Coward A (1998) Phyto-estrogen: where are we now? Brit J Nutr 79:393–406
Bradley JM, Davis KM, Deroles SC, Bloor SJ, Lewis DH (1998) The maize Lc regulatory gene up-regulates the flavonoid biosynthetic pathway of Petunia. Plant J 13:381–392
Bradley JM, Deroles SC, Boase, Bloor S, Swinny E, Davis KM (1999) Variation in the ability of the maize Lc regulatory gene to upregulate flavonoid biosynthesis in heterologous systems. Plant Sci 140:31–39
Bravo L (1998) Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev 56:317–333
Burbulis I, Iacobucci M, B Shirley (1996) A null mutation in the first enzyme of flavonoid biosynthesis does not affect male fertility in Arabidopsis. Plant Cell 8:1013–1025
Cassidy A, Bingham S (1995) Biological effects of isoflavones in young women: importance of the chemical composition of soybean products. Brit J Nutr 74:587–601
Davies K, Bloor S, Spiller G (1998) Production of yellow colour in flowers: redirection of flavonoid biosynthesis in Petunia. Plant J 13:259–266
Davis MS, Solbiati J, Cronan, Jr JE (2000) Overproduction of acetyl-CoA carboxylase activity increases the rate of fatty acid biosynthesis in Escherichia coli. J Biol Chem 275:28593–28598
Davis SR, Dalais FS, Simpson ER, Murkias AL (1999) Phytoestrogens in health and disease. Recent Prog Horn Res 54:185–210
Dixon RA, Steele CL (1999) Flavonoids and isoflavonoids – a gold mine for metabolic engineering. Trends Plant Sci 4:394–400
Ferrer JL, Jez JM, Bowman ME, Dixon RA, Noel JP (1999) Structure of chalcone synthase and the molecular basis of plant polyketide biosynthesis. Nat Struct Biol 6:775–784
File SE, Jarrett N, Fluck E, Duffy R, Casey K, Wiseman H (2001) Eating soya improves human memory. Psychopharmacology 157:430–436
Fischer R, Budde I, Hain R (1997) Stilbene synthase gene expression causes changes in flower colour and male sterility in tobacco. Plant J 11:489–498
Forkmann G, Martens S (2001) Metabolic engineering and applications of flavonoids. Curr Opin Biotechnol 12:155–160
Frits WA, Coward L, Wang J, CA Lamartiniere (1998) Dietary genistein: prenatal mammary cancer prevention, bioavailability and toxicity testing in rat. Carcinogenesis 19:2152–2158
Fulda M, Heinz E, FP Wolter. (1994) The fadD gene of Escherichia coli K-12 is located close to rnd at 39.6 min of the chromosomal map and is a new member of the AMP-binding protein family. Mol Gen Genet 242:241–249
Galati G, Teng S, Moridani MY, Chan TS, O'Brien PJ (2000) Cancer chemoprevention and apoptosis mechanisms induced by dietary polyphenolics. Drug Methabol Drug Interact 17:311–349
Grayer RJ, Harborne JB (1994) A survey of antifungal compounds from higher plants, 1982–1993. Phytochemistry 37:19–42
Grotewold E, Chamberlin M, Snook M, Siame B, Butler L, Swenson J, Maddock S, St Clair G, Bowen B (1998) Engineering secondary metabolism in maize cells by ectopic expression of transcription factors. Plant Cell 10:721–740
Hahlbrock K, Grisebach H (1979) Enzymatic controls in the biosynthesis of lignin and flavonoids. Annu Rev Plant Physiol 30:105–130
Hahlbrock K, Zilg H, Grisebach H (1970) Stereochemistry of the enzymatic cyclisation of 4,2′,4′-trihydroxychalcone to 7,4′-dihydroxyflavanone by isomerases from mung bean seedlings. Eur J Biochem 15:13–18
Harborne JB (1999) The comparative biochemistry of phytoalexin induction in plants. Biochem Syst Ecol 27:335–368
Hollman PC, Katan MB (1997) Absorption, metabolism and health effects of dietary flavonoids in man. Biomed Pharmacother 51:305–310
Horinouchi S,Beppu T (1994) A-factor as a microbial hormone that controls cellular differentiation and secondary metabolism in Streptomyces griseus. Mol Microbiol 12:859–864
Hotze M, Schröder G, Schröder J (1995) Cinnamate 4-hydroxylase from Cantharanthus roseus, and a strategy for functional expression of plant cytochrome P450 proteins as translational fusions with P450 reductase in E. coli. FEBS Lett 374:345–350
Hwang EI, Kaneko M, Ohnishi Y, Horinouchi S (2003) Production of plant-specific flavanones by Escherichia coli containing an artificial gene cluster. Appl Environ Microbiol 69:2699–2706
Jez JM, Austin MB, Ferrer JL, Bowman ME, Schröder J, Noel JP (2000) Structural control of polyketide formation in plant-specific polyketide synthase. Chem Biol 7:919–930
Jez JM, Noel JP (2002) Reaction mechanism of chalcone isomerase. J Biol Chem 277:1361–1369
Kaneko M, Ohnishi Y, Horinouchi S (2003) Cinnamate:coenzyme A ligase from the filamentous bacterium Streptomyces coelicolor A3(2). J Bacteriol 185:20–27
Knobloh KH, Hahlbrock K (1975) Isoenzymes of p-coumarate:CoA ligase from cell suspension of Glycine max. Eur J Biochem 52:311–320
Knott J, Martens S, Forkmann G (2000) Induction of resistance mechanism against fungal infection of cultivars of Rosa. Polyphenols Commun 2:627–628
Kreuzaler F, Hahlbrock K (1975) Enzymic synthesis of an aromatic ring from acetate units. Eur J Biochem 56:205–213
Kyndt JA, Meyer TE, Cusanovich MA, Van Beeumen JJ (2002) Characterization of a bacterial tyrosine ammonia lyase, a biosynthetic enzyme for the photoactive yellow protein. FEBS Lett 512:240–244
Lamartiniere CA (2000) Protection against breast cancer with genistein: a component of soy. Am J Clin Nutr 71:1705S-1707S
Lee D, Douglas CJ (1996) Two divergent members of a tobacco 4-coumarate:coenzyme A ligase (4CL) gene family. Plant Physiol 112:193–205
Le Marchand L (2002) Cancer preventive effects of flavonoids – a review. Biomed Pharmacother 56:296–301
MerzDemlow BE, Duncan AM, Wangen KE, Xu X, Carr TP, Phipps WR, Kurzer MS (2000) Soy isoflavones improve plasma lipids in normocholesterolemic, premenopausal women. Am J Clin Nutr 71:1462–1469
Messina MJ (1999) Legumes and soybeans: overview of their nutritional profiles and health effects. Am J Clin Nutr 70:439S-450S
Mo Y, Nagel C, Taylor L (1992) Biochemical complementation for chalcone synthase mutants defines a role for flavonols in functional pollen. Plant Biol 89:7213–7217
Mol J, Grotewold E, Koes R (1998) How genes paint flowers and seeds. Trends Plant Sci 3:212–217
Mol JNM, Robbins MP, Dixon RA, Veltkamp E (1985) Spontaneous and enzymic rearrangement of naringenin chalcone to flavanone. Phytochemistry 24:2267–2269
Moustafa E, Wong E (1967) Purification and properties of chalcone-flavanone isomerase from soya bean seed. Phytochemistry 6:625–632
Pfeifer BA, Admiraal SJ, Gramajo H, Cane DE, Khosla C (2001) Biosynthesis of complex polyketides in a metabolically engineered strain of E. coli. Science 291:1790–1792
Pfeifer BA, Khosla C (2001) Biosynthesis of polyketides in heterologous hosts. Microbiol Mol Biol Rev 65:106–118
Pompon D, Louerat B, Bronine A, Urban P (1996) Yeast expression of animal and plant P450s in optimized redox environments. Methods Enzymol 272:51–64
Pueppke JL (1996) The genetics and biochemical basis for nodulation of legumes by rhizobia. Crit Rev Biotechnol 16:1-51
Quattrocchio F, Wing J, van der Woude K, Souer E, de Vetten N, Mol J, Koes R (1999) Molecular analysis of the anthocyanin 2 gene of petunia and its role in the evolution of flower color. Plant Cell 11:1433–1444
Rösler J, Krekel F, Amrhein N, Schmid J (1997) Maize phenylalanine ammonia-lyase has tyrosine ammonia-lyase activity. Plant Physiol 113:175–179
Schröder J (1997) A family of plant-specific polyketide synthases: facts and predictions. Trends Plant Sci 2:373–378
Schwinn K, Markham K, Given N (1994) Floral flavonoids and the potential for pelargonidin biosynthesis in commercial Chrysanthemum cultivars. Phytochemistry 35:145–150
Scott DA, Hammond PM, Brearly GM, Price CP (1992) Identification by high-performance liquid chromatography of tyrosine ammonia-lyase activity in purified fraction of Phaseolus vulgaris phenylalanine ammonia-lyase. J Chromatog B 573:309–312
Setchell KD (1998) Phytoestrogens: the biochemistry, physiology, and implications for human health of soy isoflavones. Am J Clin Nutr 68:1333S-1346S
Setchell KD, Cassidy A (1999) Dietary isoflavones: biological effects and relevance to human health. J Nutr 129:758S-767S
Stuible HP, Büttner D, Ehlting J, Hahlbrock K, Kombrink E (2000) Mutational analysis of 4-coumarate:CoA ligase identifies functionally important amino acids and verifies its relationship to other adenylate-forming enzymes. FEBS Lett 467:117–122
Takamura Y, Nomura. G (1988) Changes in the intracellular concentration of acetyl-CoA and malonyl-CoA in relation to the carbon and energy metabolism of Escherichia coli K12. J Gen Microbiol 134:2249–2253
Taylor LP, Jorgensen R (1992) Conditional male fertility in chalcone synthase-deficient Petunia. J Hered 83:11–17
Van Rhijn R, Vanderleyden J (1995) The Rhizobium-plant symbiosis. Microbiol Rev 59:124–142
van der Meer I, Stam M, van Tunen A, Mol J, Stuitje R (1992) Antisense inhibition of flavonoid biosynthesis in Petunia anthers results in male sterility. Plant Cell 4:253–262
Volpin H, Elkind Y, Okon Y, Kapulnik Y (1994) A vesicular arbuscular mycorrhizal fungus (Glomus intraradix) induces a defense response in alfalfa roots. Plant Physiol 104:683–689
Wang HK, Xia Y, Yang ZY, Natschke SL, Lee KH (1998) Recent advances in the discovery and development of flavonoids and their analogues as antitumor and anti-HIV agents. Adv Exp Med Biol 439:191–225
Weisshaar B, Jenkins GI (1998) Phenylpropanoid biosynthesis and its regulation. Curr Opin Plant Biol 1:251–257
Welle R, Schröder G, Schilts E, Grisbach H, Schröder J(1991) Induced plant responses to pathogen attack: analysis and heterologous expression of the key enzyme in the biosynthesis of phytoalexins in soybean (Glycine max L. Merr. cv. Harosoy 63). Eur J Biochem 196:423–430
Yamaguchi T, Kurosaki F, Suh DY, Sankawa U, Nishioka M, Akiyama T, Shibuya M, Ebizuka Y (1999) Cross-reaction of chalcone synthase and stilbene synthase overexpressed in E. coli. FEBS Lett 460:457–461
Zawada RJX, Khosla C (1999) Heterologous expression, purification, reconstitution and kinetic analysis of an extended type II polyketide synthase. Chem Biol 6:607–615
Acknowledgements
The work from this laboratory was supported by a Research Grant of the Noda Institute for Scientific Research, by the Bio Design Program of the Ministry of Agriculture, Forestry, and Fisheries of Japan (BDP-03-VI-2–2), and by a grant from the Industrial Technology Research Grant Program 2000 of the New Energy and Industrial Technology Development Organization of Japan (00A03004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Sir David Hopwood.
Rights and permissions
About this article
Cite this article
Kaneko, M., Hwang, E.I., Ohnishi, Y. et al. Heterologous production of flavanones in Escherichia coli: potential for combinatorial biosynthesis of flavonoids in bacteria. J IND MICROBIOL BIOTECHNOL 30, 456–461 (2003). https://doi.org/10.1007/s10295-003-0061-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-003-0061-1