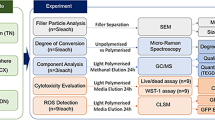
Abstract
The aim of this study was to investigate potential cellular responses and biological effects of new generation dental composites on cortical neuron cells in two different exposure times. The study group included five different bulk-fill flow able composites; Surefil SDR Flow, X-tra Base Flow, Venus Bulk Flow, Filtek Bulk Flow and Tetric-Evo Flow. They were filled in Teflon molds (Height: 4 mm, Width: 6 mm) and irradiated for 20 s. Cortical neuron cells were inoculated into 24-well plates. After 80% of the wells were coated, the 3 µm membrane was inserted and dental filling materials were added. The experiment was continued for 24 and 72 h. Cell viability measured by MTT assay test, total antioxidant and total oxidant status were examined using real assay diagnostic kits. The patterns of cell death (apoptosis) were analyzed using annexin V-FITC staining with flow cytometry. Β-defensins were quantitatively assessed by RT-PCR. IL-6, IL-8 and IL-10 cytokines were measured from the supernatants. All composites significantly affected analyses parameters during the exposure durations. Our data provide evidence that all dental materials tested are cytotoxic in acute phase and these effects are induced cellular death after different exposure periods. Significant cytotoxicity was detected in TE, XB, SS, FBF and VBF groups at 24 and 72 h, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Restorative materials with different characteristics are produced by today’s advanced technology in dentistry. It is preferred for these materials to have biocompatible on oral tissues [1,2,3,4,5]. While assessing the biological effects of restorative materials, the detrimental effects of these materials should be identified [6].
The composites used in dentistry consist of different conventional methacrylates such as bisphenol A-glycidyl methacrylate (Bis-GMA), triethylene glycol dimethacrylate (TEGDMA), urethane dimethacrylate (UDMA) and hydroxyethyl methacrylate (HEMA). These monomers are the major organic structures of the composite resins [7,8,9,10,11,12].
The clinical success of dental materials strongly based on their biological compatibility [13]. The cytotoxicity of dental composite materials has been ascribed to residual monomers [14]. These released monomers are correlated with the soft tissues in the oral cavity and the dentin-pulp system in the cavity [7,8,9,10,11,12].
Recently, in vitro and in vivo studies showed that monomers have been interrelated with genotoxicity [14,15,16,17,18,19], estrogenicity [14, 17, 19, 20], immune system [19, 21, 22], hypersensitivity, cytotoxicity [14, 15, 23, 24] and the production of reactive oxygen species [23, 25]. Based on these literature data, it is necessary to know biological and chemical effects of dental materials on cells. For this purpose nine different biological parameters were analyzed in this study in detail.
Human teeth have a large neurosensory system that is predominantly provided by the sensorial nerve fibers of trigeminal ganglion. Sensory nerve fibers progress from the tooth apex towards the enamel and innervates the surrounding tooth pulp [26,27,28]. Dense nerve fiber network in the pulp-dentin interface generate the raschkow subodontoblastic plexus and these nerve endings are provided to progress radially throughout the odontoblastic layer [29]. These sensorial nerves mediate nociception and forms the basis of a toothache. Problems in neuron cells occur and permanently damage the cell. The total number of neuron cells in the pulp is very important for tooth protection and immune defense [30].
Cytotoxicity in the restorative materials causes the formation of cellular stress [31, 32]. The formation of cellular stress leads to the observation of free radicals, formation of apoptosis and tissue destructions [31, 33,34,35,36,37,38,39]. The presence of Bcl-2 in the neuronal cells indicates that these proteins appear to inhibit apoptosis. The proteins of the Bcl-2 heterodimerize and homodimerize with each other, and the relative proportions of these gene families define whether or not a cell becomes apoptotic [40]. When a cell goes through apoptosis, viability and functionality are not possible of a tooth [41]. Thus, in the present study the effect of restorative materials on Bcl-2 gene expression on neuron cells was assessed.
Human beta defensins (HBDs) suppress proinflammatory cytokines. They activate the mast cells and perform degranulation. HBDs regulate the complement system, glucocorticoid production and antigen-specific immune response [42]. Four HBDs are isolated (HBD-1,-2,-3, and -4) until now. Although HBDs are observed in various tissues and organs, these peptides are mostly originated from epithelial cells [43, 44]. It is shown that epithelial cell-like odontoblastic cells structurally located inside the dentinal tubules of the teeth. These odontoblastic cells contain HBD-1 and HBD-2. Stimulation of odontoblast cells and microbial lipopolysaccharides with recombinant HBD-2 causes a decrease of HBD-1 and an increase of proinflammatory cytokines; IL-6 and IL-8. In addition, HBD-2 stimulates odontoblastic differentiation by increasing the dentin sialo-phosphoprotein expression [45, 46].
The studies on the cytotoxic effects of restorative materials were found in the literature review, but there was no comprehensive study assessing the biological effects of the materials. When cytotoxic effects were analyzed in the scientific studies, generally one or two parameters were investigated, but it is not enough to evaluate one or two parameters to determine the toxicity of a material. For this purpose biocompatibility tests should be supported by several parameters so that the authenticity of the research and correctness of the results increase.
In this study, we investigated potential cytotoxic and biological effects of bulk fill composites on neuron cells. We tested the viability of cells exposed to dental bulk fill composites that were widely used in dental clinics. Further, different biological parameters; total antioxidant capacity, total oxidant status, apoptosis level, IL-6, IL-8 and IL-10 cytokines, β-defensins: HBD-1 and HBD-2 were evaluated.
Materials and methods
Specimen preparation
After placing the restorative materials inside the Teflon molds (4 × 6 mm), strip bands were attached on the lower and upper surfaces of the molds and they were pressed with the glass slide to get a smooth surface. Then, sample were polymerized for 20/40 s via LED light device (Elipar Freelight II; 3 M-ESPE, St.Paul, MN, USA) according to the instructions of the producing company. After the samples were hardened, their edges and surfaces were polished using the polishing discs (Soft-Lex; 3 M ESPE, St. Paul, MN, USA). Before the materials were placed in 24-well plates; they were sterilized under UV light for 2 h. This process was repeated for all the materials used in this study. The materials used in this study are shown in Table 1.
Preparation of cell culture
Preparation of neurons
Cortical Neuron cells (Fig. 1) were supplied from Atatürk University Department of Pharmacology. The cells coming in cryofalcons were dissolved in series at normal room temperature and centrifuged for 5 min in a 1200 rpm +4 degrees centrifuge (Bachmann, Germany) by adding 2 cc NBM medium (Neurobasal Medium, Gibco, USA). The settled cells were mixed in the new medium (contains neurobasal medium, 1/10 FBS, 1/50 B27 and 0.1% antibiotic (penicillin–streptomycin-amphotericin B)), inoculated in 24-well plates and kept in the incubator containing 5% Co2 at 37°. The cells were included in the experiment when the dendrites completely covered the wells after 10 days [47].
Preparation of insert membrane well plates
Dentin tubule diameter is approximately 1 μm at the enamel-dentin border. Diameters of the dentinal tubules reach 3–4 μm on the pulp side [21]. Based on this information, insert membrane which have 3 µm pores were used in this study (Fig. 2).
The samples sterilized under UV light for 2 h were individually placed with the help of a sterile forceps to insert membranes in a sterile cabinet. Then, samples were placed into 24-well plates in which cells were located. The cells were left for incubation in the incubator of 5% CO2 at 37 °C during 24 h and 72 h (Fig. 2).
Determination of BCl-2, HBD-1, HBD-2, IL-6, IL-8, and IL-10 expression
Samples were processed blindly for the detection of mRNA transcripts of HBD-1 and HBD-2. The total cellular RNA was isolated from each sample.
RNA isolation
0.1 cc trypsin/EDTA was added to every medium of 24-well plates. After they were incubated in the incubator for 5 min, they were centrifuged at 1200 rpm during 5 min. The cells formed as a pellet and processed for RNA isolation and cDNA synthesis.
Isolation was performed using the qiagen RNA isolation kit. 1 cc Qiazole solution was added to the cells in the form of a pellet that were settled in the tube and they were kept at room temperature for 5 min. Then, 200 µl chloroform was added, agitated for 15 s, and kept at room temperature for 2–3 min. The samples were centrifuged for 15 min at the temperature of 4°. The colorless fluid at the top was transferred to another tube and it was vortexed by adding 1:1 ethanol alcohol. 700 µl of the sample was taken and placed in a collection tube and after it was closed, it was centrifuged at room temperature at 8.000g for 15 s. This stage was repeated once more. 700 µl RW1 solution was added into the RNeasy column. The cap of the column was closed and it was centrifuged at 8.000g for 15 s. Then, 500 µl RPE was centrifuged at RNeasy column at 8.000 g for 15 s. Then, 500 µl RPE was added to the RNeasy column and centrifuged at 8.000g for 2 min. After this stage, a new 1.5 cc tube was placed in the RNeasy column, 30–50 µl RNase free water was added and its cap was closed, it was centrifuged at 8.000g for 1 min.
cDNA synthesis
For the cDNA synthesis; 2 µl from the genomic DNA wipeout buffer 7 × solution and RNA 1 µg and RNase free water were prepared to have a total volume of 14 µl and after they were kept at 42° for 2 min, they were again placed in the ice. Then, a total of 20 µl including reverse transcription master mix 1 µl, Quantiscript RT buffer 5 × 4 µl, RT primer mix 1 µl and RNA 14 µl were mixed and placed in the RT-PCR device. This was heated at 42° for 15 min and at 95° for 3 min and then it was kept up to – 20°.
MTT, oxidant and antioxidant analyzes
MTT assay
Cell viability was analyzed by the MTT assay, which is based on the ability of the mitochondrial enzyme succinate dehydrogenase to convert the soluble tetrazo-lium salt (MTT) into formazan crystals in metabolically active cells. This water is stored in the cytoplasm of cells, and the color intensity is directly proportional to the amount of viable cells [48, 49].
To determine cell viability ratio of the materials, methylthiazolyldiphenyl-tetrazolium bromide (MTT) (sigma aldrich, USA) kit was applied at the end of 24 h and 72 incubation period. MTT solution (10%) was added to each well and incubated in the incubator containing 5% CO2 at 37 °C for 4 h. After 4 h medium plates removed and 100 µl DMSO (Dimethylsulfoxide) (sigma, USA) were added. The absorbance value was read at 550 nm wavelength in (optical density) a spectrophotometer device (μQuant, Bad Friedrichshall, Biotek).
Total oxidant status (TOS)
In total oxidant status (TOS) assay, the assessment is done by measuring spectrophotometrically the density of the color related to the amount of oxidants in the sample. In the present study, TOS (Total Oxidant Status) kits manufactured by Rel Assay Diagnostics® company (Turkey) were used.
The components in the kit were reactive 1 solution, reactive 2 solution, standard 1 solution, and standard 2 solution. To determine the TOS level; 500 µl Reactive 1 solution was added to the wells in which 75 µl plasma sample was present and after reading the initial absorbance value at 530 nm, 25 µl reactive 2 solution was added in the same well and second absorbance was read at 530 nm at the end of the waiting period of 10 min at room temperature. Then, we used the following formula to determine the TOS levels (mmol Trolox Equiv./L).
ΔST2 (Δstandard 2 = ST2 s reading − ST2 first reading), Δ Sample (ΔSample = Sample second reading − Sample first reading).
Principle of the total antioxidant status (TAS) measurement
In TAS assay; antioxidant capacity was determined by inhibiting formation of the 2-2′-azinobis (3-ethylbenzothiazoline 6-sulfonate = ABTS +) radical cation. In the assay process, real assay diagnostics® (Turkey) commercial kit was used.
The components of the kit were reactive 1 solution, reactive 2 solution, standard 1 solution, and standard 2 solution. To determine the TAS level; 500 µl reactive 1 solution was added in the wells containing 30 µl sample and first absorbance was read at 660 nm. Then, 75 µl reactive 2 was added to the same wells and allowed to wait at room temperature for 10 min. At the end of the waiting period, second absorbance value was read at 660 nm. Then we used the following formula to determine the TAS levels (mmol Trolox Equiv./L).
ΔST1 (Δstandard 1 = ST1 s reading − ST1 first reading), ΔST2 (Δstandard 2 = ST2 s reading − ST2 first reading), ΔSample (ΔSample = Sample second reading- Sample first reading).
Detection of apoptosis by Annexin-V binding
The AnnexinV-FITC apoptosis detection kit (Boivision) was used to identify connecting to Annexin V, which had a stable affinity for phosphatidylserine, in order to analyze apoptosis [50].
Statistical analysis
IBM SPSS Statistics 22 (IBM SPSS, Turkey) program was used for the statistical analyses to assess the results obtained in the study. To analyze the data, OneWay ANOVA (OneWay Analysis of Variance) method was used and the significance values were compared with the control group. The significance values between the groups were assessed at the levels of p < 0.05 and p < 0.001 and a significant difference was found between the groups. We used power analyzed for stablieshed sample size (12 well for each sample).
Results
MTT analysis results after 24 h
When the XB and TE maintain cell survival within 24 h only compared to the control group; degeneration was observed in SS, FBF and VBF cells and the neurons in these groups got damaged after 24 h (Fig. 3).
TAS and TOS analysis results after 24 h
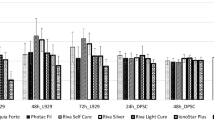
When the total antioxidant values were examined; a decrease was observed in the antioxidant status in the groups other than SS, XB and TE. TE, XB, and SS groups increased slightly more than the control group. In the other groups, antioxidant status decreased because the protection mechanism was deactivated in the cells. The cells in VBF group were more degenerated than the other groups (Fig. 4).
When the TOS values were examined; it was found that stress increased in the neurons in XB, SS, and TE groups but viability ratios were high (Fig. 5). Our data show accordance with the flow cytometry and cell viability data.
Gene expression (IL-6, IL-8, IL-10, BCL-2, HBD-1, HBD-2, and ACTB) analysis results after 24 h
While IL-6 values had an increase of 9 times in VBF group, they had an increase of 0.8-times in TE group. Similarly, IL-8 values were the highest in VBF group and the lowest in TE group. While defense cytokines (IL-10) increased in TE group, they decreased in both FBF and VBF groups. This negative decrease in BCL-2 value is an indication for their exposure to acute toxicity (Fig. 6). This decrease in BCL-2 value indicate an increase in apoptosis ratio.
MTT analysis results after 72 h
Viability ratio of the cells was higher in TE when compared with the other groups. Within 72 h, XB could not tolerate the viability ratio and a decrease of 35% was observed in the viability ratio. Degeneration ratio in TE was 21%. 72 h later, TE material maintained the cell viability at the rate of 80% (Fig. 7).
TAS analysis results after 72 h
When the MTT results in the neurons were examined, viability values were low in VBF and FBF. The antioxidant values of VBF and FBF were very low when compared with the control group and cell degeneration was high. While the antioxidant value was 2.55 in the control group, it was 1.1 in VBF group and 1.32 in FBF group. This value was the lowest in VBF group (1.1) (Fig. 8).
TOS analysis results after 72 h
When the control group was examined in terms of neurons, TOS value was found to be 1.16 and a significant increase was observed in the TOS values in other groups. Oxidant levels of the groups other than TE increased approximately 3 times than the control group. Antioxidant values increased approximately 3 times in VBF, 3 times in FBF, and 3 times in XB when compared with the control group. In other words, this 3-times increase showed that there was an excessive stress in the cells. In the present study, no cellular degeneration was observed in XB and SS groups. They were not exposed to any stress. Other than XB and SS materials; XD, FBF and VBF showed that there would be an increase in the apoptosis ratio (Fig. 9).
Gene expression (IL-6, IL-8, IL-10, BCL-2, hBD-1, hBD-2, and ACTB) analysis results after 72 h
When the genetic results of the neurons were assessed; it was found that while ACTB, HBD-1, IL-6, IL-10, BCL-2, and IL-8 genes were expressed, HBD-2 gene expressions cannot be taken. At the end of 72 h, there was an increase of 10% in almost all the genes. While IL-6 was 0.38 in TE at the end of 24 h, this value was 3.21 at the end of 72 h. Similarly, when the analysis results were assessed; while the IL-6 value in FBF was 1.98 after 24 h, it increased to 31.03 at the end of 72 h. When the analysis results were assessed; IL-8 value showed an increase at the end of 72 h. This showed that cellular breakdown was present. When the MTT results were examined, the increase in cellular degeneration over time verified the results of these parameters. When the IL-10 values were examined, no change was observed between 24 and 72 h. Because the IL-10 level is a protective mechanism, it remained constant but the increase in the inflammatory parameters signified that cell degeneration increased. While the cell ratio in TE was 97% at the end of 24 h, it was 79% after 72 h. Similarly, while the cell ratio in XB was 92% after 24 h, it decreased to 65% after 72 h. Since these results were compatible with each other, they verified the results of the study. When the BCL-2 values were examined, BCL-2 value was found to be positive only in TE group. In other groups, negative results were obtained and the genes were suppressed except for TE group. While BCL-2 value was the highest in FBF group (− 58, 79), it was the lowest in XB group (− 2.01). This parameter showed that rate of cellular death was the highest in FBF group and XB group had the lowest rate of cellular death among the other suppressed groups. When examining MTT results to verify the result, cell viability ratio at the end of 72 h was the lowest in XB group (49%). In other words, it was the group with highest cell degeneration. When the HBD-1 gene was examined, it was found that while there was an increase of 16% in SS, no increase was observed in TE group. While the cells in TE group maintained their viability, substantial cell degeneration occurred in other groups (Fig. 10).
Discussion
During last decade, the use of bulk fill composite resins which consist of monomers and additives like photoinitiators, coinitiators, photostabilizers, inhibitors and inorganic fillers has tremendously been rising [51]. It is well known that the monomers may produce cytotoxic and biological effects as a result of incomplete cure [52, 53]. These effects are needed to be known to enlarge the use of newly produced composite resins.
Dental restorative composites should protect the viability of the pulp and associated cells. They should not show inflammatory reactions on the application site, and should not damage the tissue integrity [54].
Odontoblastic layer is the first step of the defense line. The odontoblast layer is also supported by a nociceptor network branching from the sensorial regions of the tooth pulp [27]. It is shown that bacterial pathogens activate the nociceptors forming one of the essential mechanisms causing neurogenic inflammation and pain [55]. As a response to harmful stimuli; the sensorial nerves in the tooth pulp are exposed to neuroplastic changes including the germination of terminal branches [27, 56]. In the cases that dentinal tubule infection is still limited, nociceptor germination shows itself by early nerve reaction against dentin damage [27]. In fact, the cross transition between the nervous system and the immune system is required for an effective defense against invasive situations [56]. It is shown that the sensorial denervation of the molar teeth of rats reduces the recovery ratio in dental pulp tissue and triggers a significant decrease in the chemotaxis of the immunity cells after the dentin damage [57]. Early neuroimmune response should be taken into consideration for the development of new approaches in dental treatments., otherwise, the deterioration of the condition will cause degeneration in the nerve cells and the accumulation of myelin debris in the pulp is inevitable in such cases. Peripheral nerve damage generally results in progressive infiltration of the immunocompetent cells, as the myelin debris accumulation is an obstacle in the recovery of axons and also is a major contributor to the inflammatory response after peripheral nerve damage [58]. Especially the myelin debris leads the macrophages and leukocytes mediating the clearance of tissue residues not to reach that site [59, 60].
Inadequate polymerization of the dental composite resins and resin-based bonding agents cause the free resin monomers stay in the resin matrix of the materials. These released resin monomers are correlated with the soft tissues in the oral cavity and the dentin pulp system in the cavity. In vivo and in vitro studies on the cytotoxic effect of residual monomers with associated tissues was proved by researchers [7,8,9,10,11,12, 61]. While assessing the biological effects of restorative materials, it is required to determine the harmful effects of the substances inside the material at first. This assessment can be done using the in vitro cytotoxic tests, genotoxic tests and gene suppression tests on the cell layers [62,63,64]. Cytotoxic effects on the neurons after the polymerization of new-developed composite materials can cause irreversible degenerations. Thus, the effects of the newly developed composites on the neuron lines in various periods of time were investigated by using different techniques in the present study.
There are studies using direct contact method to assess the cytotoxic effects of materials [65]. However, except for the MTT analysis, the insert membrane technique was used in the present study by simulating the dentinal tubules rather than direct contact of the cells with restorative materials. In this technique, membranes with pores of 3 micrometers are used and so, direct contact of the restorative materials with the cells is prevented. Thus, in the present in vitro study, a system that simulated dentin and allowed the diffusion of the test materials such as dentin was used, and thus the clinical status was imitated.
The cytotoxicity of a resin-based restorative material is deal with the amount of filler ratio and residual monomers disentangle from the materials. It is indicated that these monomers releasing from the resin matrix may cause biological and cytotoxic effects on dental cells over time [66, 67]. As a result of these literature research, the contents of the materials used in the present study were evaluated from cytotoxicity. When examining the filler ratio, it was observed that the least filler content was present in VBF and FBF groups. In this study, it was observed that VBF and FBF caused a more toxic effect in neurons and more degeneration when compared with the other materials. The decrease in the filler content made us think that it caused an increase in the material toxicity.
Recent work has indicated that in the groups, which have high molecular weight of ethoxylated bisphenol A dimethacrylate (BisEMA), is a matrix type of composite materials and more toxic effect was observed in these materials [68]. Similarly in the present study, the material contents of the groups were examined and it was observed that BisEMA was high especially in FBF and XB groups having high toxicity. Also similar to the results of the study assessing the effects of composites containing epoxy resin on inflammatory factors such as IL-6, IL-8, and TNF-α [69]; in this study the cytotoxic effects of flowable composites on neurons were investigated by assessing the cytokines, toxic effects were observed in epoxy resin groups and it was concluded that there was an increase in inflammatory factors.
It was reported that the contact period was another effective factor on the cytotoxicity of the materials. [19, 65]. But, in the present study, cortical neuron cells are used instead of rat pulp cells [50]. Previously, cytotoxic effects of the materials on human pulp stem cells via MTT analysis by using Venus bulk flow, Filtek supreme and Surefil SDR flow were assessed and as a result of this study the materials showed a slight cytotoxic effect [70].
In this study, SDR bulk-fill flow able composite showed a low toxicity in pulp neurons and cell viability ratio was found as 98% after 24 h. It was concluded that the materials caused a cellular degeneration of approximately 40% in the neurons after 72 h. In the present study, especially the toxic effect occurring within the first 24 h of the application of composite materials increased over time and a cell degeneration of 40% was observed in the other groups except for TE and XB groups [65, 71]. Glutamate toxicity is important in the degeneration of neurons. When the glutamate toxicity increases, positive ions start to penetrate into the cell. This degeneration can be tolerated when the negative ions penetrate into the cell as well as the entering positive ions. When TAS and TOS parameters were examined, it was observed that oxidant levels were tolerated because the high antioxidant status suppressed cell stress and cellular degeneration, and thus there was viability in the neurons.
IL-6 plays an important role in the defense mechanism and inflammatory processes [72]. IL-6 is secreted by cells, involved in host defense against infectious microorganisms and products, and damaged tissues [73]. IL-6 improves B cell differentiation and antibody secretion, T-cell proliferation, acute-phase protein synthesis, and the upregulation of phospholipase A2 [74]. Interleukin 6 also play a central role in gingival and periodontal inflammation [75]. It is desirable that the filler materials used in dental clinical trials should not induce IL-6 cytokine. In this study, an increase in IL-6 levels was observed in all groups after 72 h. After 72 h, IL-6 level was the least in the TE group whereas IL-6 level was the highest in the XB group. The percentage of fillers in both groups was found to be almost similar, so, it can be considered that matrix type is more effective than filler ratio of this difference. The level of IL-6 in the SS group (highest filler content) at the end of 72 h confirms that the increase in IL-6 level is more related to the type of matrix.
In this study, it is reported that Human β-defensins play an important role in the development of odontoblasts, which are responsible for formation of dentin, an agent responsible for local host defense and minor gene expression has been detected in HBD-2 in dental pulp cells in undecayed teeth and uninfected teeth [45].
Paris et al. investigated that while HBD-2 and HBD -3 were weakly expressed in healthy and inflammatory pulps, hBD -1 and hBD -4 significantly increased in inflammation compared to healthy pulps. They found that hBD-1 and hBD-4 played a role in pulpal host defense [76]. In this study; HBD-1 and HBD-2 were not expressed after 24 h in the neurons. After 72 h, the HBD-1 was expressed; whereas, HBD-2 was still not expressed. These expressions were not observed in the neurons because there were no HBD-1 and HBD-2 genes. In this study, we determined that degeneration was present in most of the cells over time. This was an indication for the fact that cellular degeneration was not caused by apoptosis, but by necrosis. The cells were directly degenerated before apoptosis. While assessing the cytotoxic effects of neuron cells, conducing the studies having an experiment period lasting for more than 72 h can provide more detailed information about the gene expression.
Conclusion
This study provides new insights in the biological effects of composites on neuron cells in vitro. The results showed that the cytotoxic effects increased over time. However, it is not exactly known that which the changes occur in cells after 72 h. Although bulk fill composite resins are being improved, their cytotoxic effects became still got disadvantageous. Recently, composites without resin monomers have been applied to the dental market. Based on these concerns, considerable effort is being expended to develop alternative monomer systems which may have fewer negative biological consequences.
Clinical relevance
Neuronal cells are responsible for the stimulation of reparative dentine and the pain sensation of teeth. Neuronal cells never grow proliferation, so the numbers of neuron cells are fixed. The reduction in neuronal cells shortens the long-term clinical course of the teeth. Therefore, it is important to evaluate the effects of composites on neuron cells instead of dental pulp and gingival fibroblast cells in cytotoxicity studies.
References
Six N, Lasfargues JJ, Goldberg M. In vivo study of the pulp reaction to Fuji IX, a glass ionomer cement. J Dent. 2000;28:413–22.
Modena KC, Casas-Apayco LC, Atta MT, et al. Cytotoxicity and biocompatibility of direct and indirect pulp capping materials. J Appl Oral Sci. 2009;17:544–54.
Brannstrom M, Vojinovic O. Response of the dental pulp to invasion of bacteria around three filling materials. ASDC J Dent Child. 1976;43:83–9.
Cox CF, Keall CL, Keall HJ, Ostro E, Bergenholtz G. Biocompatibility of surface-sealed dental materials against exposed pulps. J Prosthet Dent. 1987;57:1–8.
Grieve AR, Alani A, Saunders WP. The effects on the dental pulp of a composite resin and two dentine bonding agents and associated bacterial microleakage. Int Endod J. 1991;24:108–18.
Mousavinasab SM. Biocompatibility of composite resins. Dent Res J (Isfahan). 2011;8:S21–9.
Ortengren U, Wellendorf H, Karlsson S, Ruyter IE. Water sorption and solubility of dental composites and identification of monomers released in an aqueous environment. J Oral Rehabil. 2001;28:1106–15.
Geurtsen W, Leyhausen G. Chemical-Biological Interactions of the resin monomer triethyleneglycol-dimethacrylate (TEGDMA). J Dent Res. 2001;80:2046–50.
Moharamzadeh K, Van Noort R, Brook IM, Scutt AM. HPLC analysis of components released from dental composites with different resin compositions using different extraction media. J Mater Sci Mater Med. 2007;18:133–7.
Thonemann B, Schmalz G, Hiller KA, Schweikl H. Responses of L929 mouse fibroblasts, primary and immortalized bovine dental papilla-derived cell lines to dental resin components. Dent Mater. 2002;18:318–23.
Engelmann J, Leyhausen G, Leibfritz D, Geurtsen W. Metabolic effects of dental resin components in vitro detected by NMR spectroscopy. J Dent Res. 2001;80:869–75.
Janke V, von Neuhoff N, Schlegelberger B, Leyhausen G, Geurtsen W. TEGDMA causes apoptosis in primary human gingival fibroblasts. J Dent Res. 2003;82:814–8.
Wataha JC. Predicting clinical biological responses to dental materials. Dent Mater. 2012;28:23–40.
Goldberg M. In vitro and in vivo studies on the toxicity of dental resin components: a review. Clin Oral Investig. 2008;12:1–8.
Kleinsasser NH, Schmid K, Sassen AW, et al. Cytotoxic and genotoxic effects of resin monomers in human salivary gland tissue and lymphocytes as assessed by the single cell microgel electrophoresis (Comet) assay. Biomaterials. 2006;27:1762–70.
Kleinsasser NH, Wallner BC, Harreus UA, et al. Genotoxicity and cytotoxicity of dental materials in human lymphocytes as assessed by the single cell microgel electrophoresis (comet) assay. J Dent. 2004;32:229–34.
Schweikl H, Hiller KA, Bolay C, et al. Cytotoxic and mutagenic effects of dental composite materials. Biomaterials. 2005;26:1713–9.
Urcan E, Scherthan H, Styllou M, et al. Induction of DNA double-strand breaks in primary gingival fibroblasts by exposure to dental resin composites. Biomaterials. 2010;31:2010–4.
Longo DL, Paula-Silva FW, Faccioli LH, et al. Cytotoxicity and cytokine expression induced by silorane and methacrylate-based composite resins. J Appl Oral Sci. 2016;24:338–43.
Wada H, Tarumi H, Imazato S, Narimatsu M, Ebisu S. In vitro estrogenicity of resin composites. J Dent Res. 2004;83:222–6.
Rakich DR, Wataha JC, Lefebvre CA, Weller RN. Effect of dentin bonding agents on the secretion of inflammatory mediators from macrophages. J Endod. 1999;25:114–7.
Kostoryz EL, Tong PY, Strautman AF, et al. Effects of dental resins on TNF-alpha-induced ICAM-1 expression in endothelial cells. J Dent Res. 2001;80:1789–92.
Chang MC, Chen LI, Chan CP, et al. The role of reactive oxygen species and hemeoxygenase-1 expression in the cytotoxicity, cell cycle alteration and apoptosis of dental pulp cells induced by BisGMA. Biomaterials. 2010;31:8164–71.
Wisniewska-Jarosinska M, Poplawski T, Chojnacki CJ, et al. Independent and combined cytotoxicity and genotoxicity of triethylene glycol dimethacrylate and urethane dimethacrylate. Mol Biol Rep. 2011;38:4603–11.
Schmalz G, Krifka S, Schweikl H. Toll-like receptors, LPS, and dental monomers. Adv Dent Res. 2011;23:302–6.
Rodd HD, Boissonade FM. Comparative immunohistochemical analysis of the peptidergic innervation of human primary and permanent tooth pulp. Arch Oral Biol. 2002;47:375–85.
Byers MR, Suzuki H, Maeda T. Dental neuroplasticity, neuro-pulpal interactions, and nerve regeneration. Microsc Res Tech. 2003;60:503–15.
Monteiro J, Day P, Duggal M, Morgan C, Rodd H. Pulpal status of human primary teeth with physiological root resorption. Int J Paediatr Dent. 2009;19:16–25.
Rodd HD, Boissonade FM. Innervation of human tooth pulp in relation to caries and dentition type. J Dent Res. 2001;80:389–93.
Couve E, Osorio R, Schmachtenberg O. Reactionary Dentinogenesis and Neuroimmune Response in Dental Caries. J Dent Res. 2014;93:788–93.
Thompson EB. Apoptosis and steroid hormones. Mol Endocrinol. 1994;8:665–73.
Krifka S, Spagnuolo G, Schmalz G, Schweikl H. A review of adaptive mechanisms in cell responses towards oxidative stress caused by dental resin monomers. Biomaterials. 2013;34:4555–63.
Adams JM, Cory S. Life-or-death decisions by the Bcl-2 protein family. Trends Biochem Sci. 2001;26:61–6.
Adrain C, Martin SJ. The mitochondrial apoptosome: a killer unleashed by the cytochrome seas. Trends Biochem Sci. 2001;26:390–7.
Spierings DC, de Vries EG, Vellenga E, et al. Tissue distribution of the death ligand TRAIL and its receptors. J Histochem Cytochem. 2004;52:821–31.
Billig H, Furuta I, Rivier C, et al. Apoptosis in testis germ cells: developmental changes in gonadotropin dependence and localization to selective tubule stages. Endocrinology. 1995;136:5–12.
Estaquier J, Idziorek T, de Bels F, et al. Programmed cell death and AIDS: significance of T-cell apoptosis in pathogenic and nonpathogenic primate lentiviral infections. Proc Natl Acad Sci USA. 1994;91:9431–5.
McPhie DL, Coopersmith R, Hines-Peralta A, et al. DNA synthesis and neuronal apoptosis caused by familial Alzheimer disease mutants of the amyloid precursor protein are mediated by the p21 activated kinase PAK3. J Neurosci. 2003;23:6914–27.
Ozeki N, Mogi M, Nakamura H, Togari A. Differential expression of the Fas-Fas ligand system on cytokine-induced apoptotic cell death in mouse osteoblastic cells. Arch Oral Biol. 2002;47:511–7.
Mitsuyasu T, Harada H, Higuchi Y, et al. Immunohistochemical demonstration of bcl-2 protein in ameloblastoma. J Oral Pathol Med. 1997;26:345–8.
Slootweg PJ, de Weger RA. Immunohistochemical demonstration of bcl-2 protein in human tooth germs. Arch Oral Biol. 1994;39:545–50.
Porter EM, Liu L, Oren A, Anton PA, Ganz T. Localization of human intestinal defensin 5 in Paneth cell granules. Infect Immun. 1997;65:2389–95.
Schneider JJ, Unholzer A, Schaller M, Schafer-Korting M, Korting HC. Human defensins. J Mol Med (Berl). 2005;83:587–95.
Diamond G, Russell JP, Bevins CL. Inducible expression of an antibiotic peptide gene in lipopolysaccharide-challenged tracheal epithelial cells. Proc Natl Acad Sci USA. 1996;93:5156–60.
Dommisch H, Winter J, Acil Y, et al. Human beta-defensin (hBD-1, -2) expression in dental pulp. Oral Microbiol Immunol. 2005;20:163–6.
Dommisch H, Winter J, Willebrand C, Eberhard J, Jepsen S. Immune regulatory functions of human beta-defensin-2 in odontoblast-like cells. Int Endod J. 2007;40:300–7.
Jiang ZF, He XP, Liu CH, Yang HY. Effects of the superoxide anionic radicals to the cultured cerebral cortex neuron of newborn rat. Acta Biochim Biophys Sin. 2001;33:552–8.
Ulukaya E, Ozdikicioglu F, Oral AY, Demirci M. The MTT assay yields a relatively lower result of growth inhibition than the ATP assay depending on the chemotherapeutic drugs tested. Toxicol In Vitro. 2008;22:232–9.
Wang X, Xia Y, Liu L, et al. Comparison of MTT assay, flow cytometry, and RT-PCR in the evaluation of cytotoxicity of five prosthodontic materials. J Biomed Mater Res B Appl Biomater. 2010;95:357–64.
Liu XM, Chapman GB, Wang H, Durante W. Adenovirus-mediated heme oxygenase-1 gene expression stimulates apoptosis in vascular smooth muscle cells. Circulation. 2002;105:79–84.
Cokic SM, Hoet P, Godderis L, et al. Cytotoxic effects of composite dust on human bronchial epithelial cells. Dent Mater. 2016;32:1482–91.
Salehi S, Gwinner F, Mitchell JC, Pfeifer C, Ferracane JL. Cytotoxicity of resin composites containing bioactive glass fillers. Dent Mater. 2015;31:195–203.
Gupta SK, Saxena P, Pant VA, Pant AB. Release and toxicity of dental resin composite. Toxicol Int. 2012;19:225–34.
Council on Dental Materials and Devices. Composite restorative materials: recommended uses. J Am Dent Assoc. 1971;82:399.
Chiu IM, Heesters BA, Ghasemlou N, et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature. 2013;501:52–7.
Sakurai K, Okiji T, Suda H. Co-increase of nerve fibers and HLA-DR- and/or factor-XIIIa-expressing dendritic cells in dentinal caries-affected regions of the human dental pulp: an immunohistochemical study. J Dent Res. 1999;78:1596–608.
Fristad I, Heyeraas KJ, Kvinnsland IH, Jonsson R. Recruitment of immunocompetent cells after dentinal injuries in innervated and denervated young rat molars: an immunohistochemical study. J Histochem Cytochem. 1995;43:871–9.
Gaudet AD, Popovich PG, Ramer MS. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation. 2011;8:110.
Angelova A, Takagi Y, Okiji T, Kaneko T, Yamashita Y. Immunocompetent cells in the pulp of human deciduous teeth. Arch Oral Biol. 2004;49:29–36.
Duruturk L, Sari S, Sengul A. Immunocompetent cell level as a diagnostic reference for pulpal pathosis of primary teeth. Arch Oral Biol. 2013;58:1517–22.
Gerzina TM, Hume WR. Effect of dentine on release of TEGDMA from resin composite in vitro. J Oral Rehabil. 1994;21:463–8.
Hanks CT, Wataha JC, Sun Z. In vitro models of biocompatibility: a review. Dent Mater. 1996;12:186–93.
Murray PE, Garcia Godoy C, Garcia Godoy F. How is the biocompatibilty of dental biomaterials evaluated? Med Oral Patol Oral Cir Bucal. 2007;12:E258–66.
Schmalz G, Browne RM. The biological evaluation of medical devices used in dentistry. The influence of the European Union on the preclinical screening of dental materials. Int Dent J. 1995;45:275–8.
Fleming GJ, Awan M, Cooper PR, Sloan AJ. The potential of a resin-composite to be cured to a 4 mm depth. Dent Mater. 2008;24:522–9.
Geurtsen W. Biocompatibility of resin-modified filling materials. Crit Rev Oral Biol Med. 2000;11:333–55.
Ferracane JL, Condon JR. Rate of elution of leachable components from composite. Dent Mater. 1990;6:282–7.
Durner J, Schrickel K, Watts DC, Ilie N. Determination of homologous distributions of bisEMA dimethacrylates in bulk-fill resin-composites by GC–MS. Dent Mater. 2015;31:473–80.
Ilday NO, Celik N, Dilsiz A, et al. The effects of silorane composites on levels of cytokines and periodontal parameters. Contemp Clin Dent. 2013;4:437–42.
Marigo L, Spagnuolo G, Malara F, et al. Relation between conversion degree and cytotoxicity of a flowable bulk-fill and three conventional flowable resin-composites. Eur Rev Med Pharmacol Sci. 2015;19(23):4469–80.
Franz A, König F, Anglmayer M, et al. Cytotoxic effects of packable and nonpackable dental composites. Dent Mater. 2003;19:382–92.
Barton BE. IL-6: insights into novel biological activities. Clin Immunol Immunopathol. 1997;85:16–20.
Takahashi K, Takashiba S, Nagai A, et al. Assessment of Interleukin-6 in the Pathogenesis of Periodontal-Disease. J Periodontol. 1994;65:147–53.
Dongari-Bagtzoglou AI, Ebersole JL. Production of inflammatory mediators and cytokines by human gingival fibroblasts following bacterial challenge. J Periodontal Res. 1996;31:90–8.
Murakami S, Hino E, Shimabukuro Y, et al. Direct interaction between gingival fibroblasts and lymphoid cells induces inflammatory cytokine mRNA expression in gingival fibroblasts. J Dent Res. 1999;78:69–76.
Paris S, Wolgin M, Kielbassa AM, Pries A, Zakrzewicz A. Gene expression of human beta-defensins in healthy and inflamed human dental pulps. J Endod. 2009;35:520–3.
Acknowledgement
This research was supported by the authors’ institutions (Fırat University Scientific Research Center/Project ID: DHF.16.02).
Funding
The work was supported by Fırat University Scientific Research Center, Elazıg, Turkey. (Project ID: DHF.16.02).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article contain studies with cortical neuron cells. All procedures performed in studies involving cortical neuron cells were in accordance with ethical standards of Firat University Non-Interventional Ethics committee; Date: 19.07.2016; Decision No: 02.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kamalak, H., Kamalak, A., Taghizadehghalehjoughi, A. et al. Cytotoxic and biological effects of bulk fill composites on rat cortical neuron cells. Odontology 106, 377–388 (2018). https://doi.org/10.1007/s10266-018-0354-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-018-0354-5