Abstract
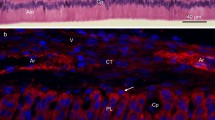
In an effort to identify a possible role for type III collagen in the morphogenesis of circumvallate papillae on the surface of the rat tongue, we examined its appearance by fluorescent immunostaining, in conjunction with differential interference contrast images and images obtained, after staining with toluidine blue, in the transmission mode by laser-scanning microscopy. We analyzed semi-ultrathin sections of epoxy resin-embedded samples of the lingual mucosa of embryonic and juvenile rats, 13 days after conception (E13) to day 21 after birth (P21). Immunoreactivity specific for type III collagen was recognized first in the mesenchymal connective tissue just beneath the circumvallate papilla placode in fetuses on E13. At this stage, most of the lingual epithelium with the exception of the circumvallate papilla placode was pseudostratified epithelium composed of one or two layers of cuboidal cells. However, the epithelium of the circumvallate papilla placode was composed of several layers of cuboidal cells. Immunoreactivity specific for type III collagen was detected mainly on the lamina propria just beneath the lingual epithelium of the rudiment of the circumvallate papilla and the developing circumvallate papilla in fetuses on E15 and E17, and slight immunostaining was detected on the lamina propria around the rudiment. In fetuses on E19, immunoreactivity specific for type III collagen was widely and densely distributed on the connective tissue around the developing circumvallate papillae and, also, on the connective tissue that surrounded the lingual muscle. However, the immunoreactivity specific for type III collagen was sparsely distributed on the lamina propria of each central papillar structure. After birth, from P0 to P14, morphogenesis of the circumvallate papillae advanced gradually with the increase in the total volume of the tongue. At these postnatal stages, the intensity of the fluorescence due to immunoreactivity specific for type III collagen was distinctively distributed on the lamina propria around each circumvallate papilla, on each central bulge and on the connective tissue that surrounded the lingual muscle. However, immunofluorescence was less distinct on the connective tissue that surrounded the lingual muscle. Thus, type III collagen appeared in conjunction with the morphogenesis of the circumvallate papillae, as well as in the connective tissue that surrounded the lingual muscle during myogenesis of the rat tongue.





Similar content being viewed by others
References
Iwasaki S, Yoshizawa H, Kawahara I. Study by scanning electron microscopy of the morphogenesis of three types of lingual papilla in the rat. Anat Rec. 1997;247:528–41.
Iwasaki S, Yoshizawa H, Kawahara I. Study by scanning electron microscopy of the morphogenesis of three types of lingual papilla in the mouse. Acta Anat. 1996;157:41–52.
Iwasaki S, Okumura Y, Kumakura M. Ultrastructural study of the relationship between the morphogenesis of filiform papillae and the keratinization of the lingual epithelium in the mouse. Cells Tissues Organs. 1999;165:91–103.
Iwasaki S, Yoshizawa H, Kawahara I. Ultrastructural study of the relationship between the morphogenesis of filiform papillae and the keratinization of the lingual epithelium in the rat. J Anat. 1999;195:27–38.
Iwasaki S, Aoyagi H, Yoshizawa H. Immunohistochemical detection of the expression of keratin 14 in the lingual epithelium of rats during the morphogenesis of filiform papillae. Arch Oral Biol. 2003;48:605–13.
Iwasaki S, Yoshizawa H, Aoyagi H. Immunohistochemical expression of keratins 13 and 14 in the lingual epithelium of rats during the morphogenesis of filiform papillae. Arch Oral Biol. 2006;51:416–26.
Garrone R, Lethias C, Guellec D. Distibution of minor collagens during skin development. Microsc Res Tech. 1997;38:407–12.
Lochner K, Gaemlich A, Südel KM, Venzke K, Moll I, Knott A, Stäb F, Wenck H, Döring O, Böttger M, Gallinat S. Expression of decorin collagens I and III in different layers of human skin in vivo: a laser capture microdissection study. Biogerontology. 2006;8:269–82.
Risau W, Lemmon V. Changes in the vascular extracellular matrix during embryonic vasculogenesis and angiogenesis. Dev Biol. 1988;125:441–50.
Nicosia RF, Villaschi S. Autoregulation of angiogenesis by cells of the vessel wall. Int Rev Cytol. 1999;185:1–43.
Anstrom JA, Thore CR, Moody DM, Challa VR, Blocks SM, Brown WR. Morphometric assessment of collagen accumulation in germinal matrix vessels of premature human neonates. Neuropathol Appl Neurobiol. 2005;31:181–90.
Reddi AH, Gay R, Gay S, Miller EJ. Transitions in collagen types during matrix-induced cartilage, bone, and bone marrow formation. Proc Natl Acad Sci USA. 1977;74:5589–92.
Carter DH, Sloan P, Aaron E. Immunolocalization of collagen types I, III tenascin, and fibronectin in intramembranous bone. J Histochem Cytochem. 1991;39:599–606.
Cournil I, Leblond CP, Pomponio J, Hand AR, Sederlof L, Martin GR. Immunohistochemical localization of procollagens. I. Light microscopic distribution of procollagen I, III and IV antigenicity in the rat incisor tooth by the indirect peroxidase anti-peroxidase method. J Histochem Cytochem. 1979;27:1059–69.
Tung PS, Domenicucci C, Wasi S, Sodek J. Specific immunohistochemical localization of osteonectin and collagen type I and III in fetal and adult porcine dental tissue. J Histochem Cytochem. 1985;33:531–40.
Takita K, Ohsaki Y, Nakata M, Kurisu K. Immunofluorescence localization of type I and type III collagens and fibronectin in mouse dental tissue in late development and during molar eruption. Arch Oral Biol. 1987;32:273–9.
Xu LX, Ohsaki Y, Nagata K, Kurisu K. Immunohistochemical studies on the distribution and age-related changes of type I and III collagen in the oral mucosa of mice. J Dent Res. 1993;72:1336–43.
Iwasaki S, Aoyagi H, Yoshizawa H. Immunohistochemical detection of epidermal growth factor and epidermal growth factor receptor in the lingual mucosa of rats during the morphogenesis of filiform papillae. Acta Histochem. 2007;109:37–44.
Mayor HD, Hampton JC, Rosario B. A simple method for removing the resin from epoxy-embedded tissue. J Cell Biol. 1961;9:909–10.
Shi S-R, Key ME, Karla KL. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhanced method for immunohistochemical staining based on microwave heating of tissue sections. J Histochem Cytochem. 1991;39:741–8.
Mbiene JP, Roberts JD. Distribution of keratin 8-containing cell clusters in mouse embryonic tongue: Evidence for a prepattern for taste bud development. J Comp Neurol. 2003;457:111–22.
Linsenmayer TF. Collagen. In: Hay ED, editor. Cell biology of extracellular matrix. New York: Plenum; 1981. p. 5–37.
Keene DR, Sakai LY, Bächinger HP, Burgeson RE. Type III collagen can be present on banded collagen fibrils regardless of fibril diameter. J Cell Biol. 1987;105:2393–402.
Iwasaki S, Asami T, Wanichanon C, Yoshizawa H, Aoyagi H. Immunohistochemical analysis of type III collagen expression in the lingual mucosa of rats during organogenesis of the tongue. Odontology. 2008;96:12–20.
Aoyagi H, Asami T, Yoshizawa H, Wanichanon C, Iwasaki S. Newly developed technique for dual localization of keratins 13 and 14 by fluorescence immunohistochemistry. Acta Histochemistry. 2008;110:324–32.
Jonker L, Kist R, Aw A, Wappler I, Peters H. Pax9 is required for filiform papilla development and suppresses skin-specific differentiation of the mammalian tongue epithelium. Mech Develop. 2004;121:1313–22.
Iwasaki S, Aoyagi H, Asami T. Expression of keratin 18 in the periderm cells of the lingual epithelium of fetal rats: visualization by fluorescence immunohistochemistry and differential interference contrast microscopy. Odontology. 2006;94:64–8.
Acknowledgments
The authors are grateful to Professor T. Kimura, Department of Oral Pharmacology, The Nippon Dental University School of Life Dentistry at Niigata, for his encouragement and helpful support. This research was supported by Research Promotion Grants (nos. NDUF-07-01, NDUF-07-11 and NDUF-08-18) from the Nippon Dental University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iwasaki, Si., Yoshizawa, H. & Aoyagi, H. Localization of type III collagen in the lingual mucosa of rats during the morphogenesis of circumvallate papillae. Odontology 100, 10–21 (2012). https://doi.org/10.1007/s10266-011-0020-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-011-0020-7