Abstract
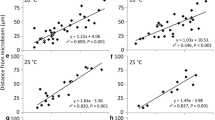
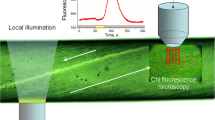
Chloroplasts change their intracellular positions in response to their light environment. Under darkness, chloroplasts assume special positions that are different from those under light conditions. Here, we analyzed chloroplast dark positioning using Adiantum capillus-veneris gametophyte cells. When chloroplasts were transferred into darkness, during the first 1–5 h, they moved towards the anticlinal cell walls bordering the adjacent cells rather rapidly. Then, they slowed down and accumulated at the anticlinal walls gradually over the following 24–36 h. The chloroplast movements could be roughly classified into two different categories: initial rapid straight movement and later, slow staggering movement. When the chloroplast accumulation response was induced in dark-adapted cells by partial cell irradiation with a microbeam targeted to the center of the cells, chloroplasts moved towards the beam spot from the anticlinal walls. However, when the microbeam was switched off, they moved to the nearest anticlinal walls and not to their original positions if they were not the closest, indicating that they know the direction of the nearest anticlinal wall and do not have particular areas that they migrate to during dark positioning.






Similar content being viewed by others
References
Araki T, Komeda Y (1993) Flowering in darkness in Arabidopsis thaliana. Plant J 4:801–811
Baum G, Long JC, Jenkins GI, Trewavas AJ (1999) Stimulation of the blue light phototropic receptor NPH1 causes a transient increase in cytosolic Ca2+. Proc Natl Acad Sci USA 96:13554–13559
Borthwick HA, Hendrick SB, Parker MW, Toole EH, Toole VK (1952) A reversible photoreaction controlling seed germination. Proc Natl Acad Sci USA 38:662–666
Chory J, Chatterjee M, Cook RK, Elich T, Fankhauser C, Li J, Nagpal P, Neff M, Pepper A, Poole D, Reed J, Vitart V (1996) From seed germination to flowering, light controls plant development via the pigment phytochrome. Proc Natl Acad Sci USA 93:12066–12071
Corchnoy SB, Swartz TE, Lewis JW, Szundi I, Briggs WR, Bogomolni RA (2003) Intramolecular proton transfers and structural changes during the photocycle of the LOV2 domain of phototropin 1. J Biol Chem 278:724–731
Darwin C, Darwin F (1880) The power of movement in plants. John Murray, London
DeBlasio SL, Mullen JL, Luesse DR, Hangarter RP (2003) Phytochrome modulation of blue light-induced chloroplast movements in Arabidopsis. Plant Physiol 133:1471–1479
Harada A, Sakai T, Okada K (2003) Phot1 and phot2 mediate blue light-induced transient increases in cytosolic Ca2+ differently in Arabidopsis leaves. Proc Natl Acad Sci USA 100:8583–8588
Hayama R, Coupland G (2003) Shedding light on the circadian clock and the photoperiodic control of flowering. Curr Opin Plant Biol 6:13–19
Iino M (2001) Phototropism in higher plants. In: Häder D, Lebert M (eds) Photomovement. ESP comprehensive series in photosciences, vol 1. Elsevier Science, London, pp 659–811
Ishikawa R, Tamaki S, Yokoi S, Inagaki N, Shinomura T, Takano M, Shimamoto K (2005) Suppression of the floral activator Hd3a is the principal cause of the night break effect in rice. Plant Cell 17:3326–3336
Iwabuchi K, Sakai T, Takagi S (2007) Blue light-dependent nuclear positioning in Arabidopsis thaliana leaf cells. Plant Cell Physiol 48:1291–1298
Kadota A, Wada M (1992) Reorganization of the cortical cytoskeleton in tip-growing fern protonemal cells during phytochrome-mediated phototropism and blue light-induced apical swelling. Protoplasma 166:35–41
Kadota A, Yamada N, Suetsugu N, Hirose M, Saito C, Shoda K, Ichikawa S, Kagawa T, Nakano A, Wada M (2009) Short actin-based mechanism for light-directed chloroplast movement in Arabidopsis. Proc Natl Acad Sci USA 106:13106–13111
Kagawa T, Wada M (1993) Light-dependent nuclear positioning in prothallial cells of Adiantum capillus-veneris. Protoplasma 177:82–85
Kagawa T, Wada M (1994) Brief irradiation with red or blue light induces orientational movement of chloroplasts in dark-adapted prothallial cells of the fern Adiantum. J Plant Res 107:389–398
Kagawa T, Wada M (1995) Polarized light induces nuclear migration in prothallial cells of Adiantum capillus-veneris L. Planta 196:775–780
Kagawa T, Wada M (1999) Chloroplast-avoidance response induced by high-fluence blue light in prothallial cell of the fern Adiantum capillus-veneris as analyzed by microbeam irradiation. Plant Physiol 119:917–923
Kagawa T, Wada M (2000) Blue light-induced chloroplast relocation in Arabidopsis thaliana as analyzed by microbeam irradiation. Plant Cell Physiol 41:84–93
Kagawa T, Sakai T, Suetsugu N, Oikawa K, Ishiguro S, Kato T, Tabata S, Okada K, Wada M (2001) Arabidopsis NPL1: a phototropin homologue controlling the chloroplast high-light avoidance response. Science 291:2138–2141
Kagawa T, Kasahara M, Abe T, Yoshida S, Wada M (2004) Function analysis of phototropin2 using fern mutants deficient in blue light-induced chloroplast avoidance movement. Plant Cell Physiol 45:416–426
Kami C, Lorrain S, Hornitschek P, Fankhauser C (2010) Light-regulated plant growth and development. Curr Top Dev Biol 91:29–66
Kasahara M, Kagawa T, Oikawa K, Suetsugu N, Miyao M, Wada M (2002a) Chloroplast avoidance movement reduces photodamage in plants. Nature 420:829–832
Kasahara M, Swartz TE, Olney MA, Onodera A, Mochizuki N, Fukuzawa H, Asamizu E, Tabata S, Kanegae H, Takano M, Christie JM, Nagatani A, Briggs WR (2002b) Photochemical properties of the flavin mononucleotide-binding domains of the phototropins from Arabidopsis, rice, and Chlamydomonas reinhardtii. Plant Physiol 129:762–773
Kawai H, Kanegae T, Christensen S, Kiyosue T, Sato Y, Imaizumi T, Kadota A, Wada M (2003) Responses of ferns to red light are mediated by an unconventional photoreceptor. Nature 421:287–290
Kodama Y, Tsuboi H, Kagawa T, Wada M (2008) Low temperature-induced chloroplast relocation mediated by a blue light receptor, phototropin 2, in fern gametophytes. J Plant Res 121:441–448
Koop HU, Schmid R, Heunert HH, Milthaler B (1978) Chloroplast migration: a new circadian rhythm in Acetabularia. Protoplasma 97:301–310
Luesse DR, DeBlasio SL, Hangarter RP (2010) Integration of phot1, phot2, and phyB signalling in light-induced chloroplast movements. J Exp Bot 61:4387–4397
Nozue K, Kanegae T, Imaizumi T, Fukuda S, Okamoto H, Yeh KC, Lagarias JC, Wada M (1998) A phytochrome from the fern Adiantum with features of the putative photoreceptor NPH1. Proc Natl Acad Sci USA 95:15826–15830
Redei GP, Acedo G, Gavazzi G (1974) Flower differentiation in Arabidopsis. Stadler Symp 6:135–168
Sakai T, Kagawa T, Kasahara M, Swartz TE, Christie JM, Briggs WR, Wada M, Okada K (2001) Arabidopsis nph1 and npl1: blue-light receptors that mediate both phototropism and chloroplast relocation. Proc Natl Acad Sci USA 98:6969–6974
Salomon M, Christie JM, Knieb E, Lempert U, Briggs WR (2000) Photochemical and mutational analysis of the FMN-binding domains of the plant blue light receptor, phototropin. Biochemistry 39:9401–9410
Senn G (1908) Die Gestalts- und Lageveränderung der Pflanzen-Chromatophoren. Wilhelm-Engelmann, Leipzig
Stoerzle S, Kagawa T, Wada M, Hedrich R, Dietrich P (2003) Blue light activates calcium-permeable channels in Arabidopsis mesophyll cells via the phototropin signaling pathway. Proc Natl Acad Sci USA 100:1456–1461
Suetsugu N, Wada M (2007) Chloroplast photorelocation movement mediated by phototropin family proteins in green plants. Biol Chem 388:927–935
Suetsugu N, Kagawa T, Wada M (2005a) An auxilin-like J-domain protein, JAC1, regulates phototropin-mediated chloroplast movement in Arabidopsis. Plant Physiol 139:151–162
Suetsugu N, Mittmann F, Wagner G, Hughes J, Wada M (2005b) A chimeric photoreceptor gene, NEOCHROME, has arisen twice during plant evolution. Proc Natl Acad Sci USA 102:13705–13709
Sugai M, Furuya M (1967) Photomorphogenesis in Pteris vittata I. Phytochrome-mediated spore germination and blue light interaction. Plant Cell Physiol 8:737–748
Swartz TE, Corchnoy SB, Christie JM, Lewis JW, Szundi I, Briggs WR, Bogomolni RA (2001) The photocycle of a flavin-binding domain of the blue light photoreceptor phototropin. J Biol Chem 276:36493–36500
Thomas B, Vince-Prue D (1997) Photoperiodism in plants. Academic Press, London
Tsuboi H, Wada M (2010) Speed of signal transfer in the chloroplast accumulation response. J Plant Res 123:381–390
Tsuboi H, Wada M (2011) Chloroplast can move in any direction to avoid strong light. J Plant Res 124:201–210
Tsuboi H, Suetsugu N, Wada M (2006) Negative phototropic response of rhizoid cells in the fern Adiantum capillus-veneris. J Plant Res 119:505–512
Tsuboi H, Suetsugu N, Kawai-Toyooka H, Wada M (2007) Phototropins and neochrome1 mediate nuclear movement in the fern Adiantum capillus-veneris. Plant Cell Physiol 48:892–896
Tsuboi H, Yamashita H, Wada M (2009) Chloroplasts do not have a polarity for light-induced accumulation movement. J Plant Res 122:131–140
Wada M, Furuya M (1978) Effects of narrow-beam irradiations with blue and far-red light on the timing of cell division in Adiantum gametophytes. Planta 138:85–90
Wada M, Kadota A, Furuya M (1983) Intracellular localization and dichroic orientation of phytochrome in plasma membrane and/or ectoplasm of a centrifuged protonema of fern Adiantum capillus-veneris L. Plant Cell Physiol 24:1441–1447
Yatsuhashi H, Wada M (1990) High-fluence rate responses in the light-oriented chloroplast movement in Adiantum protonemata. Plant Sci 68:87–94
Acknowledgments
This work was partially supported by the Japanese Ministry of Education, Sports, Science and Technology (MEXT 13139203 and 17084006 to M.W.) and the Japan Society for the Promotion of Science (JSPS 13304061, 16107002 and 20227001 to M.W.).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10265_2011_433_MOESM1_ESM.mov
Supplemental movie M1. This time-lapse movie shows chloroplast dark positioning from which the data of Figs. 3 and 4 were obtained. Images were acquired at 5-min intervals. (MOV 6158 kb)
Supplemental movie M2. This time-lapse movie shows chloroplast accumulation movement induced by blue microbeam irradiations and dark positioning from which the data of Fig. 5 were obtained. Images were acquired at 5-min intervals. (MOV 2817 kb)
Rights and permissions
About this article
Cite this article
Tsuboi, H., Wada, M. Chloroplasts move towards the nearest anticlinal walls under dark condition. J Plant Res 125, 301–310 (2012). https://doi.org/10.1007/s10265-011-0433-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-011-0433-y