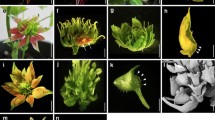
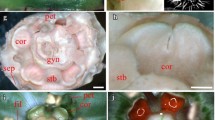
Abstract
In order to improve our understanding of floral size control we characterised three mutants of Antirrhinum majus with different macroscopic floral phenotypes. The recessive mutant compacta ähnlich has smaller flowers affected mainly in petal lobe expansion, the dominant mutant Grandiflora has overall larger organs, whilst the semidominant mutation Nitida exhibits smaller flowers in a dose-dependent manner. We developed a cell map in order to establish the cellular phenotypes of the mutants. Changes in organ size were both organ- and region-specific. Nitida and compacta ähnlich affected cell expansion in proximal and distal petal regions, respectively, suggesting differential regulation between petal lobe regions. Although petal size was smaller in compacta ähnlich than in wild type, conical cells were significantly bigger, suggesting a compensation mechanism involved in petal development. Grandiflora had larger cells in petals and increased cell division in stamens and styles, suggesting a relationship between genes controlling organ size and organ identity. The level of ploidy in petals of Grandiflora and coan was found to be equivalent to wild type petals and leaves, ruling out an excess of growth via endoreduplication. We discuss our results in terms of current models about control of lateral organ size.




Similar content being viewed by others
References
Anastasiou E, Lenhard M (2007) Growing up to one’s standard. Curr Opin Plant Biol 10:63–69
Anastasiou E, Kenz S, Gerstung M, MacLean D, Timmer J, Fleck C, Lenhard M (2007) Control of plant organ size by KLUH/CYP78A5-dependent intercellular signaling. Dev Cell 13:843–856
Armbruster WS, Di Stilio V, Tuxill JD, Flores TC, Velasquez Runk JV (1999) Covariance and decoupling of covariance of floral and vegetative traits in nine neotropica plants: a reevaluation of Berg’s correlation-pleiades concept. Am J Bot 86:39–55
Bayo-Canha A, Delgado-Benarroch L, Weiss J, Egea-Cortines M (2007) Artificial decrease of leaf area affects inflorescence quality but not floral size in Antirrhinum majus. Sci Hortic 113:383–386
Beemster GT, Fiorani F, Inze D (2003) Cell cycle: the key to plant growth control? Trends Plant Sci 8:154–158
Berg RL (1959) A general evolutionary principle underlying the origin of developmental homeostasis. Am Nat 93:103–105
Bradley D, Carpenter R, Sommer H, Hartley N, Coen E (1993) Complementary floral homeotic phenotypes result from opposite orientations of a transposon at the plena locus of Antirrhinum. Cell 72:85–95
Brewer PB, Howles PA, Dorian K, Griffith ME, Ishida T, Kaplan-Levy RN, Kilinc A, Smyth DR (2004) PETAL LOSS, a trihelix transcription factor gene, regulates perianth architecture in the Arabidopsis flower. Development 131:4035–4045
Causier B, Cook H, Davies B (2003) An Antirrhinum ternary complex factor specifically interacts with C-function and SEPALLATA-like MADS-box factors. Plant Mol Biol 52:1051–1062
Coen ES, Meyerowitz EM (1991) The war of the whorls—genetic interactions controlling flower development. Nature 353:31–37
Comba L, Corbet SA, Hunt H, Outram S, Parker JS, Glover BJ (2000) The role of genes influencing the corolla in pollination of Antirrhinum majus. Plant Cell Environ 23:639–647
Davies B, Egea-Cortines M, de Andrade SE, Saedler H, Sommer H (1996) Multiple interactions amongst floral homeotic MADS box proteins. EMBO J 15:4330–4343
Delgado-Benarroch L, Causier B, Weiss J, Egea-Cortines M (2009) FORMOSA controls cell division and expansion during floral development in Antirrhinum majus. Planta (in press) doi:10.1007/s00425-009-0910-x
Disch S, Anastasiou E, Sharma VK, Laux T, Fletcher JC, Lenhard M (2006) The E3 ubiquitin ligase BIG BROTHER controls Arabidopsis organ size in a dosage-dependent manner. Curr Biol 16:272–279
Dudareva N, Murfitt LM, Mann CJ, Gorenstein N, Kolosova N, Kish CM, Bonham C, Wood K (2000) Developmental regulation of methyl benzoate biosynthesis and emission in snapdragon flowers. Plant Cell 12:949–961
Egea Gutierrez-Cortines M, Davies B (2000) Beyond the ABCs: ternary complex formation in the control of floral organ identity. Trends Plant Sci 5:473–478
Egea-Cortines M, Saedler H, Sommer H (1999) Ternary complex formation between the MADS-box proteins SQUAMOSA, DEFICIENS and GLOBOSA is involved in the control of floral architecture in Antirrhinum majus. EMBO J 18:5370–5379
Ferjani A, Horiguchi G, Yano S, Tsukaya H (2007) Analysis of leaf development in fugu mutants of Arabidopsis reveals three compensation modes that modulate cell expansion in determinate organs. Plant Physiol 144:988–999
Frary A, Fritz LA, Tanksley SD (2004) A comparative study of the genetic bases of natural variation in tomato leaf, sepal, and petal morphology. Theor Appl Genet 109:523–533
Fujikura U, Horiguchi G, Tsukaya H (2007) Dissection of enhanced cell expansion processes in leaves triggered by a defect in cell proliferation, with reference to roles of endoreduplication. Plant Cell Physiol 48:278–286
Galliot C, Hoballah ME, Kuhlemeier C, Stuurman J (2006a) Genetics of flower size and nectar volume in Petunia pollination syndromes. Planta 225:203–212
Galliot C, Stuurman J, Kuhlemeier C (2006b) The genetic dissection of floral pollination syndromes. Curr Opin Plant Biol 9:78–82
Gaudin V, Lunness PA, Fobert PR, Towers M, Riou-Khamlichi C, Murray JA, Coen E, Doonan JH (2000) The expression of D-cyclin genes defines distinct developmental zones in snapdragon apical meristems and is locally regulated by the Cycloidea gene. Plant Physiol 122:1137–1148
Gendreau E, Traas J, Desnos T, Grandjean O, Caboche M, Hofte H (1997) Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol 114:295–305
Grafi G, Larkins BA (1995) Endoreduplication in maize endosperm: involvement of M phase-promoting factor inhibition and induction of S phase-related kinases. Science 269:1262–1264
Griffith ME, Conceicao AD, Smyth DR (1999) PETAL LOSS gene regulates initiation and orientation of second whorl organs in the Arabidopsis flower. Development 126:5635–5644
Gutierrez C, Ramirez-Parra E, Castellano MM, del Pozo JC (2002) G(1) to S transition: more than a cell cycle engine switch. Curr Opin Plant Biol 5:480–486
Honma T, Goto K (2001) Complexes of MADS-box proteins are sufficient to convert leaves into floral organs. Nature 409:525–529
Horiguchi G, Tsukaya H (2007) Effect of mutations that stimulate cell proliferation on compensated cell enlargement induced by the angustifolia3 mutation. Plant Cell Physiol 48:S54–S54
Horiguchi G, Kim GT, Tsukaya H (2005) The transcription factor AtGRF5 and the transcription coactivator AN3 regulate cell proliferation in leaf primordia of Arabidopsis thaliana. Plant J 43:68–78
Hu Y, Xie Q, Chua NH (2003) The Arabidopsis auxin-inducible gene ARGOS controls lateral organ size. Plant Cell 15:1951–1961
Hu Y, Poh HM, Chua NH (2006) The Arabidopsis ARGOS-LIKE gene regulates cell expansion during organ growth. Plant J 47:1–9
Juenger T, Perez-Perez JM, Bernal S, Micol JL (2005) Quantitative trait loci mapping of floral and leaf morphology traits in Arabidopsis thaliana: evidence for modular genetic architecture. Evol Dev 7:259–271
Kim GT, Tsukaya H, Uchimiya H (1998) The ROTUNDIFOLIA3 gene of Arabidopsis thaliana encodes a new member of the cytochrome P-450 family that is required for the regulated polar elongation of leaf cells. Genes Dev 12:2381–2391
Kim GT, Fujioka S, Kozuka T, Tax FE, Takatsuto S, Yoshida S, Tsukaya H (2005) CYP90C1 and CYP90D1 are involved in different steps in the brassinosteroid biosynthesis pathway in Arabidopsis thaliana. Plant J 41:710–721
Kolosova N, Sherman D, Karlson D, Dudareva N (2001) Cellular and subcellular localization of S-adenosyl-l-methionine: benzoic acid carboxyl methyltransferase, the enzyme responsible for biosynthesis of the volatile ester methylbenzoate in snapdragon flowers. Plant Physiol 126:956–964
Kondorosi E, Roudier F, Gendreau E (2000) Plant cell-size control: growing by ploidy? Curr Opin Plant Biol 3:488–492
Kotilainen M, Helariutta Y, Mehto M, Pollanen E, Albert VA, Elomaa P, Teeri TH (1999) GEG participates in the regulation of cell and organ shape during corolla and carpel development in Gerbera hybrida. Plant Cell 11:1093–1104
Krizek BA (1999) Ectopic expression of AINTEGUMENTA in Arabidopsis plants results in increased growth of floral organs. Dev Genet 25:224–236
Krizek BA (2009) Making bigger plants: key regulators of final organ size. Curr Opin Plant Biol 12:17–22
Kudo N, Kimura Y (2001) Flow cytometric evidence for endopolyploidization in cabbage (Brassica oleracea L.) flowers. Sex Plant Reprod 13:279–283
Laitinen RAE, Pollanen E, Teeri TH, Elomaa P, Kotilainen M (2007) Transcriptional analysis of petal organogenesis in Gerbera hybrida. Planta 226:347–360
Martin C, Gerats T (1993) Control of pigment biosynthesis genes during petal development. Plant Cell 5:1253–1264
Martin C, Bhatt K, Baumann K, Jin H, Zachgo S, Roberts K, Schwarz-Sommer Z, Glover B, Perez-Rodrigues M (2002) The mechanics of cell fate determination in petals. Phil Trans R Soc Lond Ser B Biol Sci 357:809–813
Mizukami Y, Fischer RL (2000) Plant organ size control: AINTEGUMENTA regulates growth and cell numbers during organogenesis. Proc Natl Acad Sci USA 97:942–947
Noda K, Glover BJ, Linstead P, Martin C (1994) Flower color intensity depends on specialized cell-shape controlled by a Myb-related transcription factor. Nature 369:661–664
Perez-Rodriguez M, Jaffe FW, Butelli E, Glover BJ, Martin C (2005) Development of three different cell types is associated with the activity of a specific MYB transcription factor in the ventral petal of Antirrhinum majus flowers. Development 132:359–370
Reale L, Porceddu A, Lanfaloni L, Moretti C, Zenoni S, Pezzotti M, Romano B, Ferranti F (2002) Patterns of cell division and expansion in developing petals of Petunia hybrida. Sex Plant Reprod 15:123–132
Rijpkema A, Gerats T, Vandenbussche M (2006) Genetics of floral development in Petunia. Adv Bot Res Inc Adv Plant Pathol 44(44):237–278
Schwarz-Sommer Z, Huijser P, Nacken W, Saedler H, Sommer H (1990) Genetic-control of flower development by homeotic genes in Antirrhinum majus. Science 250:931–936
Schwarz-Sommer Z, Hue I, Huijser P, Flor PJ, Hansen R, Tetens F, Lonnig WE, Saedler H, Sommer H (1992) Characterization of the Antirrhinum floral homeotic MADS-box gene deficiens: evidence for DNA binding and autoregulation of its persistent expression throughout flower development. EMBO J 11:251–263
Sommer H, Saedler H (1986) Structure of the chalcone synthase gene of Antirrhinum majus. Mol Gen Genet 202:429–434
Sommer H, Beltran JP, Huijser P, Pape H, Lonnig WE, Saedler H, Schwarzsommer Z (1990) Deficiens, a homeotic gene involved in the control of flower morphogenesis in Antirrhinum majus—the protein shows homology to transcription factors. EMBO J 9:605–613
Stubbe H (1966) Genetik und Zytologie von Antirrhinum L. sect. Antirrhinum. Fischer, Jena
Stubbe H (1974) New mutants of Antirrhinum majus. Kulturpflanze 22:189–213
Szecsi J, Joly C, Bordji K, Varaud E, Cock JM, Dumas C, Bendahmane M (2006) BIGPETALp, a bHLH transcription factor is involved in the control of Arabidopsis petal size. EMBO J 25:3912–3920
Takeda S, Matsumoto N, Okada K (2004) RABBIT EARS, encoding a SUPERMAN-like zinc finger protein, regulates petal development in Arabidopsis thaliana. Development 131:425–434
Teitel DC, Arad SM, Birnbaum E, Mizrahi Y (1985) Growth and development of tomato fruits in vivo and in vitro. Plant Growth Regul 3:179–189
Thompson DM (1988) Systematics of Antirrhinum (Scrophulariaceae) in the New-World. American Society of Plant Taxonomists. Ann Arbor, MI
Trobner W, Ramirez L, Motte P, Hue I, Huijser P, Lonnig WE, Saedler H, Sommer H, Schwarz-Sommer Z (1992) GLOBOSA: a homeotic gene which interacts with DEFICIENS in the control of Antirrhinum floral organogenesis. EMBO J 11:4693–4704
Truernit E, Haseloff J (2008) Arabidopsis thaliana outer ovule integument morphogenesis: ectopic expression of KNAT1 reveals a compensation mechanism. BMC Plant Biol 8:35
Tsukaya H (2002) Interpretation of mutants in leaf morphology: genetic evidence for a compensatory system in leaf morphogenesis that provides a new link between cell and organismal theories. Int Rev Cytol A Surv Cell Biol 217:1–39
Tsukaya H (2005) Leaf shape: genetic controls and environmental factors. Int J Dev Biol 49:547–555
Tsukaya H (2008) Controlling size in multicellular organs: focus on the leaf. Plos Biol 6:e174
Weiss J, Delgado-Benarroch L, Egea-Cortines M (2005) Genetic control of floral size and proportions. Int J Dev Biol 49:513–525
Acknowledgments
Work on A. majus in our laboratory has been funded by Fundación Séneca de la Región de Murcia, BIOCARM and Ministerio de Educación y Ciencia AGL2007-61384. L. D-B was granted by AECI. Thanks to Perla Gómez, Izaskun Mallona and María Manchado-Rojo for comments on the manuscript. We would also like to thank two anonymous reviewers for their comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Delgado-Benarroch, L., Weiss, J. & Egea-Cortines, M. The mutants compacta ähnlich, Nitida and Grandiflora define developmental compartments and a compensation mechanism in floral development in Antirrhinum majus . J Plant Res 122, 559–569 (2009). https://doi.org/10.1007/s10265-009-0236-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-009-0236-6