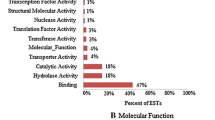
Abstract
Anthocyanin synthesis and chlorophyll degradation in regenerated torenia (Torenia fournieri Linden ex Fourn.) shoots induced by osmotic stress with 7% sucrose were examined to identify the genes regulating the underlying molecular mechanism. To achieve this, suppression subtractive hybridization was performed to enrich the cDNAs of genes induced in anthocyanin-synthesizing and chlorophyll-degrading regenerated shoots. The nucleotide sequences of 1,388 random cDNAs were determined, and these were used in the preparation of cDNA microarrays for high-throughput screening. From 1,056 cDNAs analyzed in the microarrays, 116 nonredundant genes were identified, which were up regulated by 7% sucrose to induce anthocyanin synthesis and chlorophyll degradation in regenerated shoots. Of these, eight genes were selected and RNAi transformants prepared, six of which exhibited anthocyanin synthesis inhibition and/or chlorophyll degradation in their leaf discs. Notably, the RNAi transformants of the glucose 6-phosphate/phosphate translocator gene displayed inhibition both of anthocyanin synthesis and chlorophyll degradation in both leaf discs and regenerated shoots. There was also less accumulation of anthocyanin in the petals, and flowering time was shortened. The genes we identified as being up-regulated in the regenerated torenia shoots may help further elucidate the molecular mechanism underlying the induction of anthocyanin synthesis and chlorophyll degradation.






Similar content being viewed by others
References
Aida R, Shibata M (2001) Transgenic Torenia fournieri Lind. (Torenia). In: Bajaj YPS (ed) Biotechnology in agriculture and forestry, vol 48. Springer-Verlag, Berlin, pp 294–305
Alfenito MR, Souer E, Goodman CD, Buell R, Mol J, Koes R, Walbot V (1998) Functional complementation of anthocyanin sequestration in the vacuole by widely divergent glutathione S-transferases. Plant Cell 10:1135–1149
Bevan M, Bancroft I, Bent E, Love K, Goodman H, Dean C, Bergkamp R, Dirkse W, Van Staveren M, Stiekema W, Drost L, Ridley P, Hudson SA, Patel K, Murphy G, Piffanelli P, Wedler H, Wedler E, Wambutt R, Weitzenegger T, Pohl TM, Terryn N, Gielen J, Villarroel R, De Clerck R, Van Montagu M, Lecharny A, Auborg S, Gy I, Kreis M, Lao N, Kavanagh T, Hempel S, Kotter P, Entian KD, Rieger M, Schaeffer M, Funk B, Mueller-Auer S, Silvey M, James R, Montfort A, Pons A, Puigdomenech P, Douka A, Voukelatou E, Milioni D, Hatzopoulos P, Piravandi E, Obermaier B, Hilbert H, Dusterhoft A, Moores T, Jones JD, Eneva T, Palme K, Benes V, Rechman S, Ansorge W, Cooke R, Berger C, Delseny M, Voet M, Volckaert G, Mewes HW, Klosterman S, Schueller C, Chalwatzis N (1998) Analysis of 1.9 Mb of contiguous sequence from chromosome 4 of Arabidopsis thaliana. Nature 391:485–488
Chalker-Scott L (1999) Environmental significance of anthocyanins in plant stress responses. Photochem Photobiol 70:1–9
Christie PJ, Alfenito MR, Walbot V (1994) Impact of low-temperature stress on general phenylpropanoid and anthocyanin pathways: enhancement of transcript abundance and anthocyanin pigmentation in maize seedlings. Planta 194:541–549
Close DC, Beadle CL (2003) The ecophysiology of foliar anthocyanin. Bot Rev 69:149–161
Farrant JM (2000) A comparison of mechanisms of desiccation tolerance among three angiosperm resurrection plant species. Plant Ecol 151:29–39
Ferreres F, Gil MI, Castaner M, Tomas FA (1997) Barberan phenolic metabolites in red pigmented lettuce (Lactuca sativa). Changes with minimal processing and cold storage. J Agric Food Chem 45:4249–4254
Fukusaki E, Kawasaki K, Kajiyama S, An CI, Suzuki K, Tanaka Y, Kobayashi A (2004) Flower color modulations of Torenia hybrida by downregulation of chalcone synthase genes with RNA interference. J Biotechnol 111:229–240
Garbarino JE, Oosumi T, Belknap WR (1995) Isolation of a polyubiquitin promoter and its expression in transgenic potato plants. Plant Physiol 109:1371–1378
Goodman CD, Casati P, Walbot V (2004) A multidrug resistance-associated protein involved in anthocyanin transport in Zea mays. Plant Cell 16:1812–1826
Gould KS (2004) Nature’s Swiss army knife: the diverse protective roles of anthocyanins in leaves. J Biomed Biotechnol 2004:314–320
Gould KS, Neill SO, Vogelmann TC (2002) A unified explanation for anthocyanins in leaves? Adv Bot Res 37:167–192
Hale KL, McGrath SP, Lombi E, Stack SM, Terry N, Pickering IJ, George GN, Pilon-Smits EA (2001) Molybdenum sequestration in Brassica species. A role for anthocyanins? Plant Physiol 126:1391–1402
Ingvardsen C, Veierskov B (2001) Ubiquitin- and proteasome-dependent proteolysis in plants. Physiol Plant 112:451–459
Krupa Z, Baranowska M, Orzol D (1996) Can anthocyanins be considered as heavy metal stress indicators in higher plants? Acta Physiol Plant 18:147–151
Mattanovich D, Ruker F, Machado AC, Laimer M, Regner F, Steinkellner H, Himmler G, Katinger H (1989) Efficient transformation of Agrobacterium spp. by electroporation. Nucleic Acids Res 17:6747
McKown R, Kuroki G, Warren G (1996) Cold responses of Arabidopsis mutants impaired in freezing tolerance. J Exp Bot 47:1919–1925
Miki D, Shimamoto K (2004) Simple RNAi vectors for stable and transient suppression of gene function in rice. Plant Cell Physiol 45:490–495
Moon J, Parry G, Estelle M (2004) The ubiquitin-proteasome pathway and plant development. Plant Cell 16:3181–3195
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco cultures. Physiol Plant 15:473–497
Nagira Y, Ozeki Y (2004) A system in which anthocyanin synthesis is induced in regenerated torenia shoots. J Plant Res 117:377–383
Nakajima J, Tanaka Y, Yamazaki M, Saito K (2000) cDNA cloning and gene expression of anthocyanidin synthase from Torenia fournieri. Plant Biotechnol 17:331–335
Niewiadomski P, Knappe S, Geimer S, Fischer K, Schulz B, Unte US, Rosso MG, Ache P, Flugge UI, Schneider A (2005) The Arabidopsis plastidic glucose 6-phosphate/phosphate translocator GPT1 is essential for pollen maturation and embryo sac development. Plant Cell 17:760–775
Nozzolillo C, Isabelle P, Andersen OM, Abou-Zaid M (2002) Anthocyanins of jack pine (Pinus banksiana) seedlings. Can J Bot 80:796–801
Price J, Laxmi A, St Martin SK, Jang JC (2004) Global transcription profiling reveals multiple sugar signal transduction mechanisms in Arabidopsis. Plant Cell 16:2128–2150
Sasaki N, Wada K, Koda T, Kasahara K, Adachi T, Ozeki Y (2005) Isolation and characterization of cDNAs encoding an enzyme with glucosyltransferase activity for cyclo-DOPA from four o’clocks and feather cockscombs. Plant Cell Physiol 46:666–670
Seki M, Ishida J, Narusaka M, Fujita M, Nanjo T, Umezawa T, Kamiya A, Nakajima M, Enju A, Sakurai T, Satou M, Akiyama K, Yamaguchi-Shinozaki K, Carninci P, Kawai J, Hayashizaki Y, Shinozaki K (2002) Monitoring the expression pattern of around 7,000 Arabidopsis genes under ABA treatments using a full-length cDNA microarray. Funct Integr Genomics 2:282–291
Sherwin HW, Farrant JM (1998) Protection mechanisms against excess light in the resurrection plants Craterostigma wilmsii and Xerophyta viscose. Plant Growth Regul 24:203–210
Shimanuki M, Shimamura K, Hirai S, Nishiuchi T, Suzuki K, Kodama H (2005) Polyethylene glycol-mediated enhancement of the hybridization rate on cDNA microarrays. Anal Biochem 344:284–286
Takahashi S, Seki M, Ishida J, Satou M, Sakurai T, Narusaka M, Kamiya A, Nakajima M, Enju A, Akiyama K, Yamaguchi-Shinozaki K, Shinozaki K (2004) Monitoring the expression profiles of genes induced by hyperosmotic, high salinity, and oxidative stress and abscisic acid treatment in Arabidopsis cell culture using a full-length cDNA microarray. Plant Mol Biol 56:29–55
Tamari G, Borochov A, Atzorn R, Weiss D (1995) Methyl jasmonate induces pigmentation and flavonoid gene expression in petunia corollas: a possible role in wound response. Physiol Plant 94:45–50
van Dijken AJ, Schluepmann H, Smeekens SC (2004) Arabidopsis trehalose-6-phosphate synthase 1 is essential for normal vegetative growth and transition to flowering. Plant Physiol 135:969–977
Villadsen D, Smith SM (2004) Identification of more than 200 glucose-responsive Arabidopsis genes, none of which responds to 3-O-methylglucose or 6-deoxyglucose. Plant Mol Biol 55:467–477
Yanovsky MJ, Kay SA (2002) Molecular basis of seasonal time measurement in Arabidopsis. Nature 419:308–312
Yazaki K (2005) Transporters of secondary metabolites. Curr Opin Plant Biol 8:301–307
Yoshida S (2003) Molecular regulation of leaf senescence. Curr Opin Plant Biol 6:79–84
Acknowledgments
We thank Prof. Ko Shimamoto (Nara Institute of Science and Technology) for the kind donation of pANDA35HK. We also thank Dr. Ryutaro Aida and Dr. Michio Shibata (National Institute of Floricultural Science) for providing torenia plants. Part of this work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nagira, Y., Shimamura, K., Hirai, S. et al. Identification and characterization of genes induced for anthocyanin synthesis and chlorophyll degradation in regenerated torenia shoots using suppression subtractive hybridization, cDNA microarrays, and RNAi techniques. J Plant Res 119, 217–230 (2006). https://doi.org/10.1007/s10265-006-0264-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-006-0264-4